
ProTrials Research, Inc. and Calithera Biosciences have reached an agreement for early phase clinical trial support.

ProTrials Research, Inc. and Calithera Biosciences have reached an agreement for early phase clinical trial support.


Bioclinica announces the acquisition of Orlando, Florida based Compass Research.
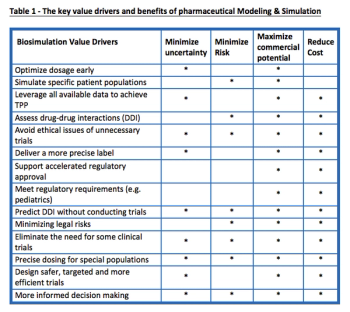
The drug development process has been seen as inefficient and disappointing by those in the R&D space. Model and Simulation provides a potential relief to these issues by delivering significant business, scientific and clinical value to drug developers.

The European Medicines Agency is proposing changes to the current guidelines on first-on-human clinical trials to improve risk-based strategies.


Medidata announces a collaboration with New York’s Memorial Sloan Kettering Cancer Center on a pilot study to expand the use of mHealth technology in oncology treatment.

The European Medicines Agency (EMA) is proposing changes to the current guidelines on first-in-human clinical trials to improve risk-based strategies. This proposal was made with cooperation from the European Commission and the Member States of the European Union (EU).


Certara announces the addition of Mitsubishi Tanabe to its Simcyp Consortium as the group’s 34th biopharmaceutical company.

ClinCapture hosts a panel discussion with experts from CROs and sponsor companies to discuss conducting quality clinical trials in a cost effective manner.

OmniComm Systems announces a three-year agreement with Montreal-based CRO JSS Medical Research.


A recent survey was conducted by Worldwide Clinical Trials at DIA 2016 on June 26-30, 2016 in Philadelphia, PA.

To provide more targeted training and validate understanding to employees, it is important to establish baseline knowledge. This case study explores how Chiltern developed its Medical Device Body of Knowledge training program.

Continuous Glucose Monitoring (CGM) devices have the ability to replace the traditional finger-prick to measure glucose levels in a patient’s blood. Quintiles’ device expert, Sam Osman, explains CGM and how the FDA decision could affect clinical trials.


Medidata announces the launch of Medidata Payments, an extension of the company’s Clinical Cloud platform.


This recent report explores key factors behind customer satisfaction and other dynamics within the Late Phase market.


Wingspan Technology announces the release of Wingspan eREG to their Life Sciences Solution.


The Society for Clinical Research Sites (SCRS) and ARCS Australia announce a partnership to explore collaboration and promote educational offerings.

Updated employee announcements, business news and recognition in the industry today.

OmniComm’s Keith Howells speaks to ACT about whether electronic data capture (EDC) can be considered innovative in today's pharma.

Virtual clinical trials use tech devices and social engagement platforms to conduct trials from a patient’s home. These electronic processes offer new opportunities for a patient-centric approach to clinical research.


SCORR Marketing and Applied Clinical Trials conducted a survey of trial project managers.