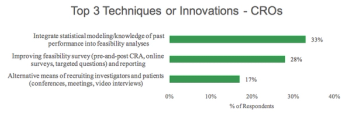
This Best Practice in Study Feasibility report covers techniques and innovations proffered by sponsors and CROs for conducting feasibility analyses.

This Best Practice in Study Feasibility report covers techniques and innovations proffered by sponsors and CROs for conducting feasibility analyses.

Antimicrobial resistance presents a challenge on a global scale that has received attention from the United Nations General Assembly among other governing bodies. Research and development for new antimicrobials and alternative medicines is needed to combat such a threat.


A critical challenge that clinical trial researchers face is patient drop out. Why patients fail to complete studies can be attributed to a variety of reasons, and delays in compensation can often be one of them.
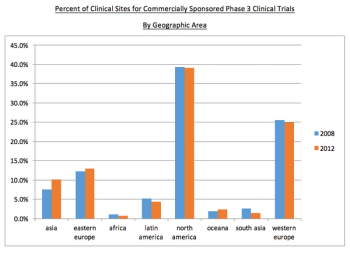
The globalization of clinical trials is the subject of this analysis to find if there is an increase in non-traditional sites.

Medidata has announced that Polaris Pharmaceuticals has selected Medidata Payments to integrate its clinical trial site reimbursement process.



Validic has published a research paper depicting the results of a 2016 Global Pharma and Biotech Survey on Digital Health.

After months of debate, clinical research activity regulated or funded by the government must adhere to revised guidelines regarding transparency. A final rule published by the Department of Health and Human Services states that all beyond Phase I FDA regulated and NIH funded clinical trials must comply with the new requirements.

ICON plc has completed its acquisition of Clinical Research Management, Inc. (ClinicalRM), a provider of full service and functional research solutions to U.S. government agencies.


ERT has announced that its electronic Clinical Outcome Assessment (eCOA) solution has assisted CoLucid Pharmaceuticals during its Phase III migraine drug study.

Clinical trials endure high drop out rates due in part to long schedules, high travel costs and long reimbursement times. Outsourcing specialized clinical trial traveling services can help alleviate this concern by allowing sponsors to efficiently manage travel and other expenses.

Pharma has been transitioning from its paper based methods of the past toward automated cloud based systems in order to offset the cost of drug development. The emphasis is now shifting toward improved study startup processes for shorter clinical trial timelines.
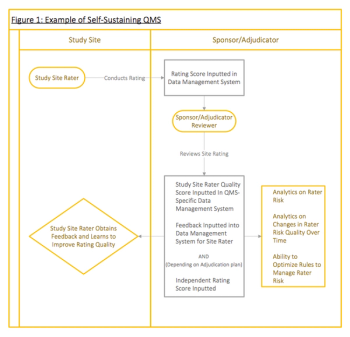
Large clinical research enterprises have the resources to establish high standard clinical trial Quality Management Systems, while smaller enterprises may not have the means to do so. This article covers how clinical research enterprises can leverage technologies to develop an efficient clinical trial QMS.


INC Research and the Center for Information and Study on Clinical Research Participation (CISCRP) have announced the finalists for their “Inspiring Hope” Ideathon event.

A key success factor for lean models is the possession of technology that can measure performance and manage risk in a well-organized and user-friendly manner. This article describes the results of an investigation to identify and describe the basic requirements for such technology solutions.

myTomorrows, an online facilitator of early access to investigational drugs, introduces an openly accessible API database relating to clinical trials.


Bracket has randomized its first patient using Precision Block Design (PBD) technology fully integrated into its clinical IRT platform and its mobile application.




The Profil Institute for Clinical Research and Antaros Medical have collaborated to apply a broad range of non-invasive PET/MRI imaging techniques into pre- and postmarketing studies of cardiometabolic drugs and drug candidates.

Medidata and the University of North Carolina Wilmington have announced a partnership to bring Medidata’s Clinical Cloud technology to the university’s classrooms.

What used to be segregated approaches in clinical trials-ePRO, telemedicine, mobile health, devices and wearables-now gather under the umbrella of technologies that touch the patient. And those technologies can have positive implications for costs efficiencies in clinical trials, streamlined data collection, as well as on patient compliance and retention.
