
inVentiv Health announced the launch of two global Site Advisory Groups (SAGs) at the annual Global Site Solutions Summit.

inVentiv Health announced the launch of two global Site Advisory Groups (SAGs) at the annual Global Site Solutions Summit.



Cincinnati-based CRO Medpace has selected Montrium's eTMF Connect and RegDocs Connect solutions.
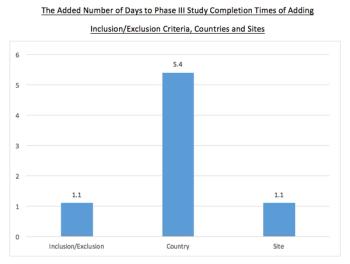
This analysis uses a large dataset to forecast the study completion time costs of specific protocol designs and execution plans.

Boston-based BBK Worldwide announced that its RSG (Ready. Set. Go.) program has improved patient recruitment and retention based on supporting data from five major global studies.


Veeva Systems has introduced the additions of Veeva Vault EDC and eSource Solutions to the Veeva Vault Clinical Suite.

Applied Clinical Trials and SCORR Marketing recently conducted this survey to gain insight on the challenges the health science industry is facing to ensure drug products and biomarker samples are getting to the right place, at the right time, in the right way. The survey report also includes information on inventory related issues, utilization of technological solutions, challenges at different points in the delivery process, drug product tracking and predictions on future trends in clinical supply chain management.


The FDA has signed a Cooperative Research and Development Agreement (CRADA) with CluePoints for its Central Statistical Monitoring solution.


SCRS and Eli Lilly have announced a Site Engagement Partnership.


Boston and Chicago-based academic research organization Alliance Foundation Trials (AFT) has selected Wingspan’s eTMF and Site Zone life science solutions.

As biopharma companies continue to explore and experience ways in which risk-based monitoring is implemented, the process of such can be misconstrued. Peter Schiemann elaborates on some of the current issues of RBM interpretation and implementation.


The EMA has appointed Grzegorz Cessak as its vice-chair of management for the next three years.


Medidata and the Society for Clinical Research Sites (SCRS) have announced the launch of a clinical trial Site Advisory Group (SAG).

Updated employee announcements, business news and recognition in the industry today.

Globalization has increasingly become a part of the clinical trial landscape as pharma sponsors conduct trials internationally. With language barriers being among the challenges of this process, translation management is now a must during the planning phase of a global trial.

Clinical research in China has shown potential as many biopharma companies look to enter the market, though the infrastructure is lacking. Peter Schiemann, PhD conveys his perspectives on the challenges that sponsors are facing in China.



PAREXEL has announced the launch of its new active tracking service.

MedAvante, Inc. announced the newest release of its Virgil Investigative Study Platform.
The Pediatric Working Group of EUCROF launched an initiative to achieve standardization of generic Informed Consent Form and Assent Form templates within European countries. The differences are noted based on a prior survey.

The need for biopharma to demonstrate the value of medical products is changing trial design in order to generate real world data. Dr. Catherine Bonuccelli of GSK discusses the Salford Lung Study, its patient-centric design and how it differs from randomized clinical trials.
