
News



Bio-Optronics Inc. has announced the release of an upgrade for Clinical Conductor CTMS, which now includes patient management for care quality.
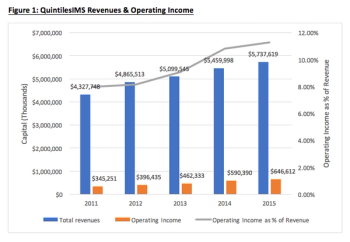
The CRO industry is experiencing exponential growth as a result of higher demand from the biopharma industry and an increased investment in R&D. This evaluation of QuintilesIMS attempts to provide some hints as to how this sector is advancing in the biopharma industry.

Updated employee announcements, business news and recognition in the industry today.



CRF Health has announced the launch and FDA clearance of its Entra BLE Smart wireless glucose meter.

Renova Therapeutics has announced the selection of Worldwide Clinical Trials as its CRO for an upcoming gene therapy study.


OmniComm Systems announces the signing of contracts to provide the company’s TrialOne clinical automation solution to three clinical research organizations in China.

The EMA has published a four-page document to summarize its main achievements relating to marketing authorizations of new medicines and the safety monitoring of authorized medicines during the past year.


Phlexglobal announces that Chapel Hill, NC-based CRO Rho has chosen their PhlexEview 4 solution as its electronic Trial Master File (eTMF) solution.

New technologies have accelerated the speed and effectiveness of clinical research and trials. This convergence is making it possible for new innovations and possibilities to bring groundbreaking treatment options to patients.

Traceability plays a crucial role in ensuring the integrity of source data and in reinforcing clinical research results. CDISC has developed Trace-XML as an extension of its Define-XML model for delivering clinical data lifestyle traceability from data collection through to final analysis.


Medidata has announced that Celgene has expanded its adoption of the Medidata Clinical Cloud to include the company’s suite of monitoring tools and data analytics solutions.


A recently completed analysis conducted by the Tufts Center for the Study of Drug Development stated that physicians and nurses only refer a fraction of their patients for clinical trials.



Brazil-based iHealth Group has joined Clinerion’s Patient Recruitment System platform as the company’s first hospital cluster in South America.

Profil Institute for Clinical Research, Inc. has announced the launch of its new corporate name, ProSciento Inc.


Interactive Response Technology solutions company, endpoint, has been chosen as the sole IRT provider for Denmark-based Ferring Pharmaceuticals AS.

Cancer Research UK has become a member of the Workforce Development Steering Committee of the Association of Clinical Research Professionals (ACRP).


Almac Clinical Technologies, part of the Almac Group, has launched Almac Clinical University, a web-based training and educational platform.

While patient centricity has been a newfound goal of the pharma industry, many would argue that adopting this philosophy has been more talk than action thus far. eClinicalHealth conducted a case study to learn what patient engagement means to industry professionals.