
News







George Clinical's Managing Director discusses the concern biopharma companies have with CFDA's slow drug and medical device approvals.




This article discusses the adoption of RBQM in academic settings and the lessons that were learned.

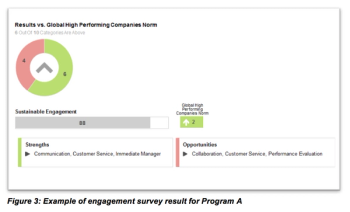
A review of a recent FSP in which the CRO partner assumed clinical monitoring and site support responsibility for more than 50 ongoing trials.



Click the title above to open the Applied Clinical Trials September/October 2017 issue in an interactive PDF format.

The National Institutes of Health (NIH) focuses on developing strategies that will encourage further research to support safe and effective therapies for pregnant and lactating women.










A look at the results of the recently completed project, which aimed to develop an holistic monitoring system via process integration to more effectively control clinical trial risks beyond isolated RBM strategies.
