
The key to moving legacy systems-electronic or paper-to the cloud: Do it in doses-and learn from those who went before.

The key to moving legacy systems-electronic or paper-to the cloud: Do it in doses-and learn from those who went before.


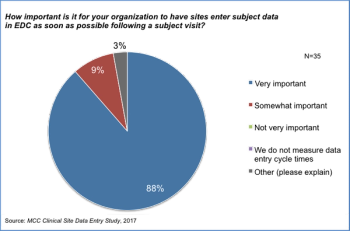
With recent reports indicating that organizations do not have a way of tracking and managing the timeliness of data entry or of site payments, The Metrics Champion Consortium wanted to see if there were opportunities to align incentives in the industry so that both sites and sponsors can achieve their goals with positive impact on clinical trials and patients.





Because of the growing rate of voice assistant applications, such as Alexa, healthcare and life sciences organizations are beginning to design a range of scenarios for deploying these voice assistant platforms in the clinical trial setting.






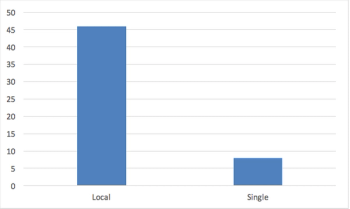
Since the emergence of the independent IRB sector, sponsors have found that regulatory and ethical review by a single IRB yields important benefits with respect to efficiency, high quality, and consistency in human research protections.

CRO's and sponsors are taking clinical trials to homes. These in-home trials can address participation issues and provide added convenience plus support for patients, but they also come with their own set of risks.

This eBook from Applied Clinical Trials features articles updating actual use studies of ePRO in a large registry trial, as well as challenges, myths, and potential useful strategies associated with “Bring Your Own Device” (BYOD) adoption.


Updated employee announcements, business news, awards, and recognition in the industry today.

This eBook will focus on the specific needs and concerns of the smaller to mid-size biopharmaceutical company. Articles cover considerations from Phase II to commercial pathways.






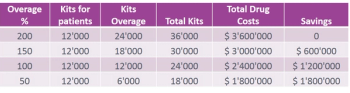
Managing clinical trial supply is not easy for the pharmaceutical industry between drug overages and right medical distribution. However, this is an important topic for sponsors to consider because the right strategy can help save money and reduce unnecessary spending.

With the ever changing regulatory landscape, outsourcing non-core activities is a flexible approach to managing resources and remaining compliant with NCO requirements for the entire generic portfolio.