
Although only the beginning of an exploratory process, the EMA is being accused of colluding in a conspiracy to favor drug firms at the expense of patient protection.

Although only the beginning of an exploratory process, the EMA is being accused of colluding in a conspiracy to favor drug firms at the expense of patient protection.

Califf tackles the many challenges to finding a balance between clinical trials large enough to assess all relevant populations and small enough to include deep data on each patient.

The European drug research consortium, IMI2, is running a program entitled Big Data for Better Outcomes Programme to promote "the evolution towards value-based and more outcomes-focused" healthcare, by generating methodologies and data that can inform policy debates.

OND Director John Jenkins would like to see sponsors invest in more “me-better” drugs-as opposed to “me-too” medicines-to expand treatment options for patients and to boost competition among manufacturers.

EMA board announces the new clinical trial rules have shifted still further away, and is now October 2018.

The burst of new technology enterprises and innovative service providers that specialize in clinical research is a sign that the clinical research industry is starting to look into new ways to solving problems culminating from an antiquated system.

Jill Wechsler on why the FDA program that encourages biopharma companies to develop new treatments for rare and neglected diseases has been in the spotlight recently.

Despite orphan-drug R&D and approval being all the rage, issues such as patient access could leave these treatments out in the rain.
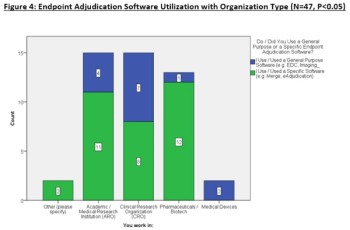
This article explores the survey data to uncover opinions about traditional endpoint adjudication use (i.e., Excel, paper, emails, etc.) compared eClinical technologies to adjudicate events.

Many of the most problematic provisions for health research were eased by amendments that provided exemptions from the overall constraints.
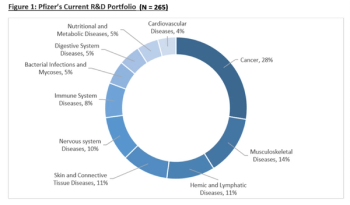
This article evaluates the impact of Allergan’s acquisition on Pfizer’s R&D Portfolio and the long term impact the acquisition will have on Pfizer’s revenue and earnings.
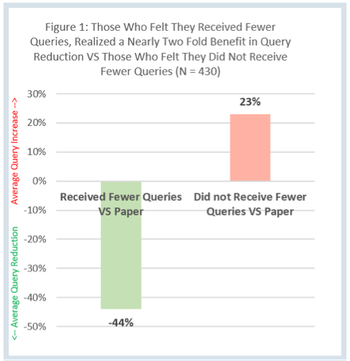
The Site Impact Survey was designed by Clinical Ink to evaluate clinical research coordinator and investigator experiences with Clinical Ink’s eSource system compared to paper source.

Nextrials CEO James Rogers believes with renewed focus on eSource driven by the FDA and industry standards groups like CDISC, sponsor companies and sites will take advantage of EHR systems to streamline clinical research.

Results from an analysis of oncology trials suggest that many patients who are excluded from participation in clinical trials due to an elevated ECG machine-generated QTc measurement may actually have been eligible for study participation.
The BMJ published a detailed report this week about how pharmaceutical sales representatives are screening people in India in return for prescriptions for their products. It also addresses the growing popularity of free “health camps” for poor people in India.
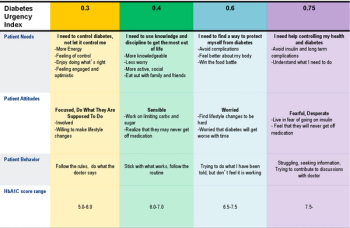

The industry is experiencing a paradigm shift from pharmacovigilance rooted in case processing and compliance reporting to a safety program built around benefit-risk management.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

Pharma and biotech companies are working with academics and health care organizations to establish systems for collecting and sharing the results of clinical trials, but they have far to go, according to a recent analysis of industry adherence to data transparency requirements. A report from the non-profit Bioethics International finds that for a group of new drugs approved by FDA in 2012, large pharma companies fell “below legal and ethical standards” for making public information from the relevant clinical trials.
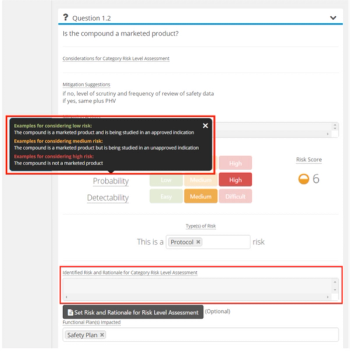
TransCelerate’s Risk Assessment and Categorization Tool (RACT) has exhibited usefulness with study teams and has guided organizations, such as Cancer Research UK (CRUK) to adopt their own RBM questionnaires

CBI’s conference proves RBM was just the beginning. Now companies are honing in on the risks and mitigations related to clinical trial data reporting.

The ability to have access to real-time information through leveraging technology combined with a strategic business perspective allows companies to implement a true quality management system.

Applied Clinical Trials is collaborating with the Clinical Endpoints Adjudication Group to conduct an industry survey to evaluate the impact of technology on endpoint adjudication processes.
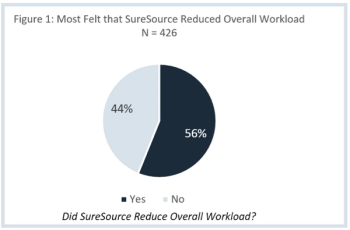
Clinical Ink, an eSource solutions provider, offered Applied Clinical Trials raw data from its Site Impact Survey for analysis. This article will delve into this data to uncover breakthrough trends regarding the utilization of eSource on workload reduction at study sites.
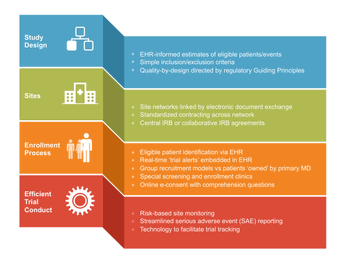
Despite the benefits of their evidence-based data, RCTs have several disadvantages.

CDER has thrown down the gauntlet to industry on eSource, and now has their work cut out for them on next steps.

In our latest webcast that aired live October 14, Oracle Health Sciences brought together three presenters from smaller or medium-size organizations in an effort to educate similar-sized companies on the benefits of EDC.

Even with the rapid increase in clinical trials and demand for cloud-based systems, doing business in China is not easy if you are unfamiliar with the territory.
Democratic candidate Hillary Clinton is introducing her Prescription Drug Plan which is meant to benefit patients, however, will Hillary’s plan be good for advancing novel therapies, and will it benefit R&D organizations?

The successful implementation of centralized monitoring requires effective planning, process restructuring, cross-functional expertise alignment, and the right technology in place.