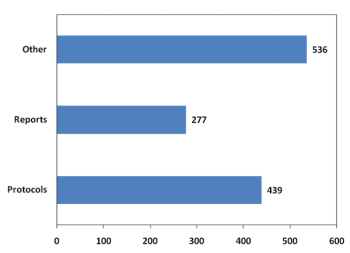Last month, Veeva Systems unveiled the results of its 2015 Paperless TMF Survey: Annual Report. The survey is in its second year, and as Jennifer Goldsmith, vice president of Veeva Vault, told Applied Clinical Trials, it was interesting and significant to report on the year-over-year results.