
The partnership is an example of how CROs and sponsors interact in the ever-evolving clinical trial industry.

The partnership is an example of how CROs and sponsors interact in the ever-evolving clinical trial industry.
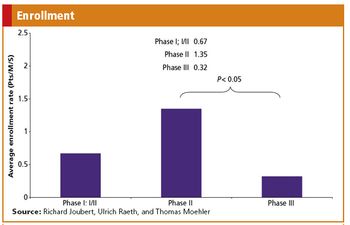
Lessons learned from the recruitment of colorectal cander patients into clinical trials.

Greater product value and innovation accomplished with sponsor-service relationships.

Five industry veterans discuss the growth, impact, and evolution of outsourcing.
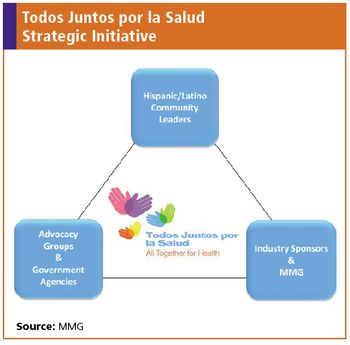
A MMG strategic initaitve to address disparities in clinical research participation among Hispanic and Latino communities.

Inappropriate calendar pacakaging is a common occurrence leading to confusion and mistakes.

Doing more harm than good will ultimately force human subject protection system reform.

Organizations are seeking growth and competitive advantage through mergers and acquistions.

Why investigative sites are at financial risk and how it may effect sponsors and CROs.
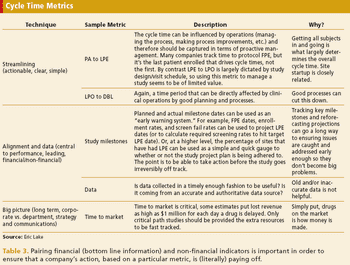
Collecting too many metrics can lead to misappropriation and misinterpretation.

A new reference guide clarifies uncertainty surrounding this sometimes misunderstood document.
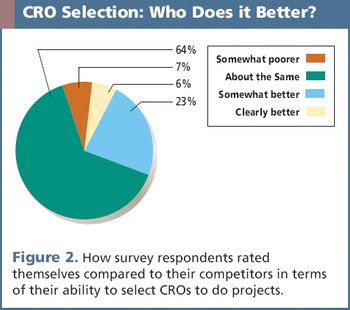
A recent survey uncovers key criteria that influence a sponsor's decision when selecting a CRO.

There is increasing recognition of the need to understand product safety in the real world.

A list of the most influential events affecting the clinical trials industry over the last 30 years.

A close-up look at these two approaches to comparator drug sourcing and how they differ.
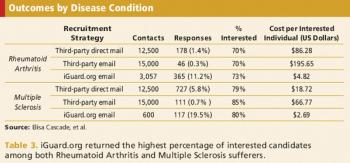
A comparison of the yields and costs of online outreach methods to other recruitment techniques.

Results reveal insight into the roles, activities, pressures, and priorities of study coordinators.
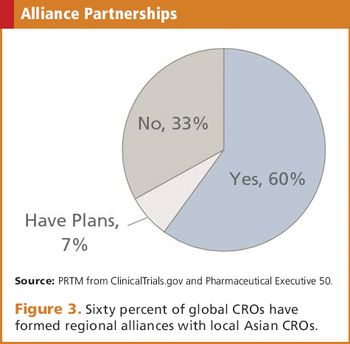
The status of the CRO industry and doing business in the most recently emerged of the Asia-Pac regions.

FDA and EMA may issue new guidelines on sponsor-CRO governance and responsibilities.

Among the many clinical development processes that need to be conducted in a smarter, more cost-effective manner, clinical data monitoring stands out as a promising area in which operational efficiencies can not only reduce costs but also improve research quality and patient safety.
An overview of the clinical research landscape in this emerging region that also looks at its challenges.

A recent survey indicates pharmacists should provide more clinical trial information to patients.

There is still an endemic inefficiency in health care and clinical trial record connectivity.
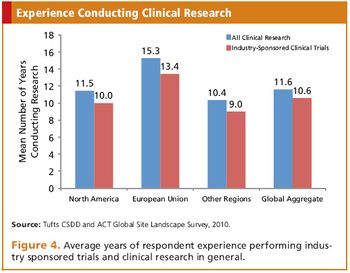
New survey from Tufts CSDD and Applied Clinical Trials provides an inside look at global sites.

Step-by-step process for budgeting global trials that uses a Currency Risk Management method.