
News




This article will focus on a case study example of a collaborative effort to share investigator, site, and study information across companies, and on the single data model that underpins it.



Krista Armstrong explains the CRO's evolving role within clinical trials.

Data integrity is the essence of GMP, the cornerstone of how the industry operates, and it is vital that all organizations embrace it to survive the rapidly changing life sciences landscape.

Updated employee announcements, business news, awards, and recognition in the industry today.

The workings of the European Union are notoriously complicated, with the result that misunderstanding is commonplace, even among those who might like to know more about it.

There have been a number of significant scientific and regulatory milestones driving the adoption of electronic patient-reported outcomes in clinical trials since the first screen-based ePRO solution, Minidoc, appeared in 1980.


Global Clinical Trial Partners (GCTP), a provider of endpoint adjudication services for clinical trials, has chosen AG Mednet’s Judi as its sole technology provider to enhance its services to its clients.

Athens Medical Group (AMG) in Greece has joined Clinerion’s hospitals network, which includes its Athens Medical Center and their Medsana Bucharest Medical Center in Romania.


Market players working on development of vaccines to fight against the virus are focused on clinical trials and look forward to gaining approval from regulatory bodies.


PAREXEL's Patient Innovation Center is launched to help sponsors navigate patient centricity from protocol, through study implementation and commercialization access.


Clinical researech technology provider Clinerion and Semicrol, the creator of Fundanet CTMS, will integrate the functionality of Clinerion’s Patient Network Explorer with Semicrol’s CTMS product.



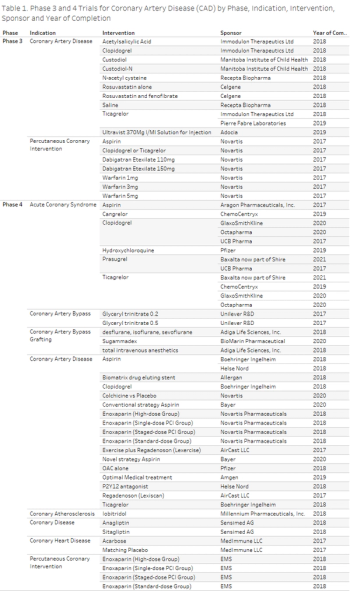
In this study, Clinical Trials Transformation Initiative’s database for aggregate analysis of ClinicalTrials.gov was used to evaluate the number of ongoing and completed interventional clinical trials for various coronary diseases.

Researchers are now looking to design their trials around a bring-your-own-device (BYOD) strategy, which allows participants in a clinical trial to provide study data using their own internet-enabled hardware.





To help combat the nation’s opioid epidemic, FDA is promoting a more tailored approach to developing and testing effective analgesics, with the aim of bringing less addictive pain treatments to market more quickly.