
A multitude of data sources leaves clinical data management systems in catch-up mode-and researcher dizzy trying to follow the data- but there is hope for simplification on the horizon.

A multitude of data sources leaves clinical data management systems in catch-up mode-and researcher dizzy trying to follow the data- but there is hope for simplification on the horizon.

Key tips and resources to help evade the common traps of market landscaping-today an increasingly high-stakes and comprehensive task during clinical development.

Click the title above to open the Applied Clinical Trials October 2018 issue in an interactive PDF format.



A new RBM method used in PaxVax trial proves successful vs. onsite source data verification for trial oversight.

In this interview, Michael J. Graziano, PhD, DABT, Vice-President, Drug Safety Evaluation for Bristol-Myers Squibb and leader of the BioCelerate Toxicology Data Sharing Initiative, provides more details about the DataCelerate platform.
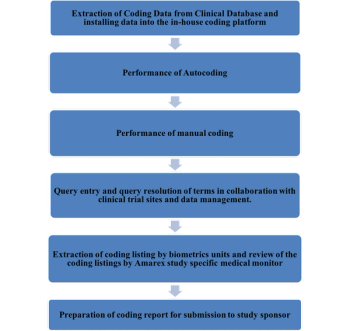
This article will detail how the Amarex Safety & Pharmacovigilance team performs medical coding using an internally developed safety platform.














The mutual recognition agreement between the European Union and the US FDA now covers 15 European countries, after Portugal won US recognition in mid-September.

This article will summarize the discussion on innovation that took place at this years Cambridge Health Institute’s Clinical Trial Innovation Summit.

These seven key building blocks for success are outlined to help companies develop and implement a data-and-analytics-driven approach to clinical trial feasibility.

A reduction of Clinical Trial Agreement Review times has caused rapid development in Malaysian clinical trials.

The critical need for new medicines to combat infectious diseases is prompting FDA to join with other federal health agencies and the biomedical research community to advance the science, regulatory policies, and reimbursement strategies to promote innovation in this area.


