
It’s time to establish standard practices to return clinical trial results summaries to patients.

It’s time to establish standard practices to return clinical trial results summaries to patients.

Europe is gradually attempting to maximize the potential of the scattered data that, if brought together, might help improve diagnosis and treatment of Europe's 30 million rare disease patients.



Reflections explored during the mid-February EFGCP conference in Brussels on making clinical research an element of better healthcare.

Sponsors have been addressing the problem with initiatives designed to find, attract, and retain patients in clinical trials more effectively.





A review of survey results looking at bridging clinical research and clinical health care.













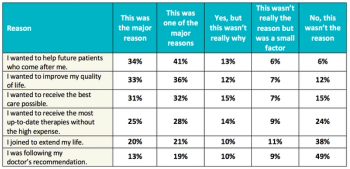
A study conducted by Antidote Technologies and SCORR Marketing looks at what patient centricity means in clinical trials and the work that remains to realize the potential for better study designs.


Database integration initiatives in the biopharmaceutical industry is now enabling clinical development departments to leverage that data for enhancing decision-making.

