
Applied Clinical Trials
FDA and industry have agreed on a set of recommendations for revising the drug user fee program, which now sits before Congress as U.S. election nears.

Applied Clinical Trials
FDA and industry have agreed on a set of recommendations for revising the drug user fee program, which now sits before Congress as U.S. election nears.

Applied Clinical Trials
New actions underway seek to find a consensus on the role of HTAs in the decision-making chain, including with clinical trials.

Applied Clinical Trials
The discussion on the main characteristics and implementation of the new Clinical Trials Regulation is heating up in Europe.
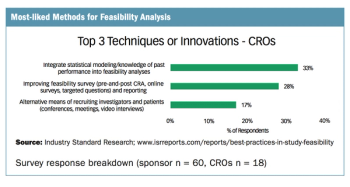
Applied Clinical Trials
Survey drills down on the preferred methods of sponsors, CROs, and sites in conducting feasibility analysis.

Applied Clinical Trials
Updated guidance incorporates the use of a patient-reported outcomes questionnaire-which could enable more endpoints and greater patient stratification for COPD trials.

Applied Clinical Trials
Q&A explores the evolution of community-based studies-and the related sample management, regulatory, compliance, and logistics support considerations.

Applied Clinical Trials
With cell-based therapies possessing multistep supply chains and complex track and trace, logistics management systems can be useful for trials even in the protocol-planning stage.

Applied Clinical Trials
New study finds that most biopharma companies are now using a standard set of key performance indicators.

Applied Clinical Trials
Study collects first comprehensive metrics on current supply management and distribution practices.

Applied Clinical Trials
An overview of clinical supply blinding methods in the context of the current research environment.
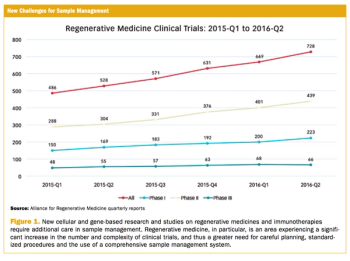
Applied Clinical Trials
From basic blood draws to more involved samples, keeping accurate track and records is crucial for trials.
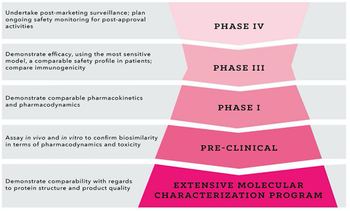
Applied Clinical Trials
Using strategic planning to address hurdles in biosimilar development programs from the outset.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials October/November 2016 issue in an interactive PDF format.