
Applied Clinical Trials
FDA's new commissioner advocates for a "learning healthcare system" and less-complex clinical trial models that tap efficiencies in study design and enrollment.

Applied Clinical Trials
FDA's new commissioner advocates for a "learning healthcare system" and less-complex clinical trial models that tap efficiencies in study design and enrollment.

Applied Clinical Trials
The agency is concerned about the lack of non-clinical models with good predictive properties in the oncology space.
Applied Clinical Trials
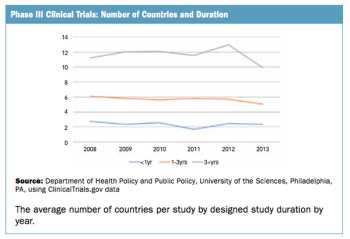
Trend doesn't seem to fall in line with the perception of an increasingly complex trial protocol climate.

Applied Clinical Trials
New policy hopes to calm the fervor around clinical trial data disclosure and confidentiality issues.

Applied Clinical Trials
Study provides real metrics on the trial-scope affects of protocol changes-shedding fresh light on the importance of adopting new strategies to reduce select amendments.

Applied Clinical Trials
For programs such as the Precision Medicine Initiative’s Cancer MoonShot to have a chance at success, following a set of key technology mandates will be critical.

Applied Clinical Trials
Pediatric trials now feature increased modeling and analytics for safer drug dosing and response.
Applied Clinical Trials
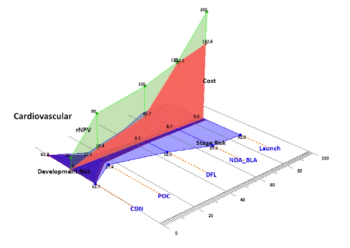
How to integrate evidence-based planning and real-world evidence to boost clinical trial productivity.

Applied Clinical Trials
How to meet the rigorous safety and efficacy demands critical to evaluating newer targeted cancer therapies.

Applied Clinical Trials
The smaller biopharmaceutical company perspective on mastering oncology immunotherapy clinical trials.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials April/May 2016 issue in an interactive PDF format.