
Clinical research in China has shown potential as many biopharma companies look to enter the market, though the infrastructure is lacking. Peter Schiemann, PhD conveys his perspectives on the challenges that sponsors are facing in China.

Clinical research in China has shown potential as many biopharma companies look to enter the market, though the infrastructure is lacking. Peter Schiemann, PhD conveys his perspectives on the challenges that sponsors are facing in China.

The need for biopharma to demonstrate the value of medical products is changing trial design in order to generate real world data. Dr. Catherine Bonuccelli of GSK discusses the Salford Lung Study, its patient-centric design and how it differs from randomized clinical trials.
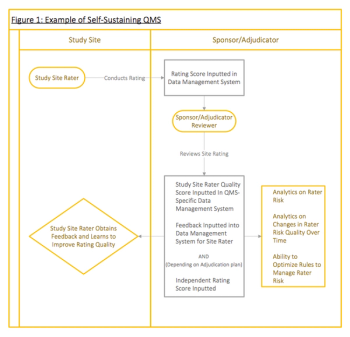
Large clinical research enterprises have the resources to establish high standard clinical trial Quality Management Systems, while smaller enterprises may not have the means to do so. This article covers how clinical research enterprises can leverage technologies to develop an efficient clinical trial QMS.

TransCelerates work on their clinical trial Quality Management Systems (QMS) initiative began about one year ago. This article summarizes findings from a recent DIA publication about the initiative and evaluates potential issues during QMS framework implementation.

TransCelerate’s Site Qualification and Training Initiative has launched a special project aimed at improving Electronic Data Capture system efficiencies between sponsors and sites. Adam Colley of Merck explains the improvements that this initiative will provide for training providers and sites.

While the clinical trials industry is currently examining mHealth technology pilots, the healthcare industry has developed them to the point of full deployment. Mon Weschler of Montefiore elaborates on his experiences successfully piloting and deploying these technologies.

TransCelerate has launched 11 informational programs through its Site Qualification and Training (SQT) initiative to improve understanding of clinical operations for new site personnel. Katarina Hugeneck of Eli Lilly and Theresa Stewart of Allergan discuss the SQT initiate with Moe Alsumidaie.

Clinical trial noncompliance has always been an industry concern. According to the FDA, the most common compliance deficiencies during inspections include inadequate investigator oversight, protocol deviations, poor record keeping, insufficient investigational product accountability, and issues with subject protection and consenting1.
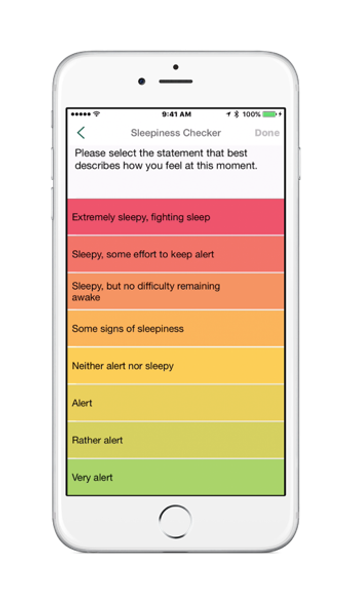
Adam Amdur of the Sleep Apnea Association speaks with Moe Alsumidaie about his company’s SleepHealth mobile app study and the impact of mHealth in clinical trials.
Successful project management requires efficiency in both identifying and addressing bottlenecks before they impact study timelines. This article examines how to design data collection criteria for optimal performance.

Using eClinical technologies to access vendor performance allows for researchers to choose appropriate vendors based on trial design, needs and risks. These decisions can be made using historical comparative data from a normative database.

Many have inquired about writing an RBM plan due to recent changes in industry execution and an increase in RBM experiences. Moe Alsumidaie responds with a brief methodology on developing a risk management process to create an RBM plan.

Pfizer has created a clinical trial modeling tool for mitigating study risk during the protocol design and study execution phases. Jonathan Rowe speaks with Moe Alsumidaie on the purpose behind these predictive models.

The CRO Forum has been asked by TransCelerate to collaborate on reviewing its initiatives. Alan Metz, Forum Chair, and Amy Kissam, Forum Vice-Chair, sit down with Moe Alsumidaie to elaborate on the CRO’s involvement.
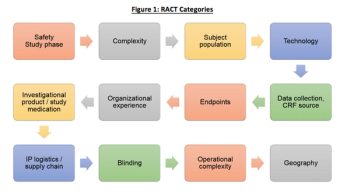
The biopharmaceutical industry is starting to adopt TransCelerate’s Risk Assessment Categorization Tool (RACT) in order to identify risks and plan a comprehensive clinical trial risk mitigation strategy. We recently wrote about the RACT moving to the cloud, and the advantages of using such systems. Some of these advantages include the ability for study teams to evaluate R&D portfolio risks by collecting and analyzing RACT data in aggregate.
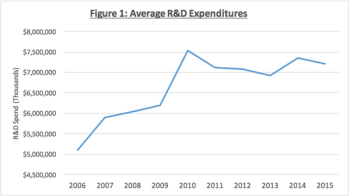
Some in the financial industry have argued that we may be in the midst of another economic recession. The biopharmaceutical industry has shown resiliency during such times and industry trends point to that being the case again.
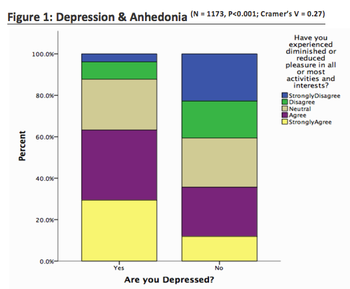
Major Depressive Disorder patients display a reduced ability to feel pleasant experiences known as Anhedonia. Such a feature provides difficulties when treating depression and engaging patients during clinical trials.

Mike Graziano, VP of Toxicology at Bristol-Myers Squibb, sits down with Moe Alsumidaie to discuss BioCelerate’s toxicology data sharing initiative.

RBM and changes in quality management are impacting study teams, CRAs and sites.
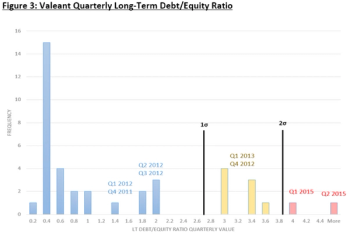
Valeant is a hedge fund disguised as pharmaceutical company, with the moral at the end of it's story, poor ethics leads to collapse.

Six TransCelerate Member Companies formed BioCelerate, a subsidiary to focus on identifying innovative solutions for operational challenges in the preclinical space.

Good clinical trial risk management involves good protocol design and study operational design at the sites.
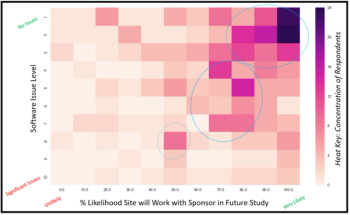
The data analysis in this article will delve into Clinical SCORE’s normative database to generate predictive models, and qualitatively reason the findings.

Using performance indicators in CRO/Vendor oversight ranges from the basic, to the unreliable to emerging innovations and strategies.
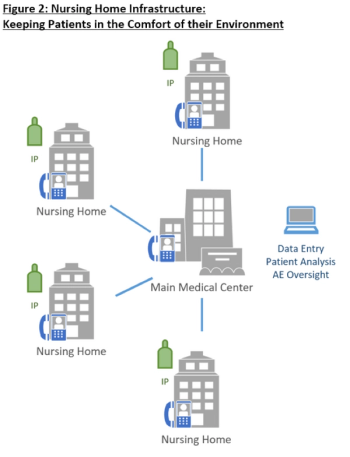
This case study describes how innovative structural design at a study site is leveraging innovative telehealth technologies and nursing home infrastructures to recruit and retain Alzheimer’s patients.
As in other needs in the clinical trials chain, endpoint adjudication is an area that can be greatly improved by technology.

The burst of new technology enterprises and innovative service providers that specialize in clinical research is a sign that the clinical research industry is starting to look into new ways to solving problems culminating from an antiquated system.
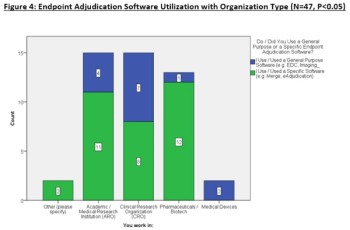
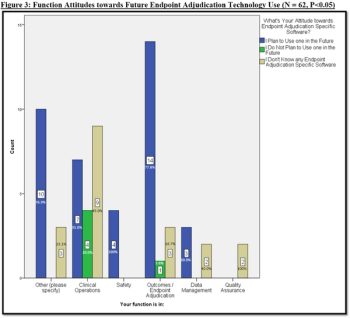
This article explores the survey data to uncover opinions about traditional endpoint adjudication use (i.e., Excel, paper, emails, etc.) compared eClinical technologies to adjudicate events.
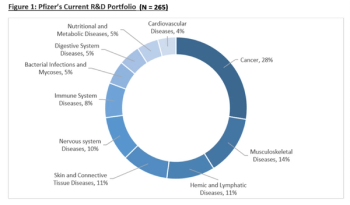
This article evaluates the impact of Allergan’s acquisition on Pfizer’s R&D Portfolio and the long term impact the acquisition will have on Pfizer’s revenue and earnings.
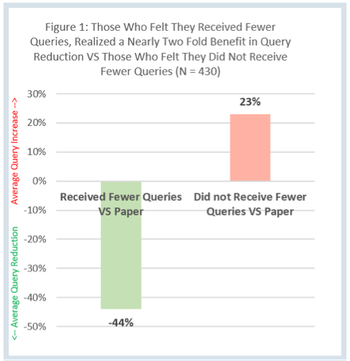
The Site Impact Survey was designed by Clinical Ink to evaluate clinical research coordinator and investigator experiences with Clinical Ink’s eSource system compared to paper source.