
Bill Tobia, CEO of Clinstruct, Ahmed Bouzid, CEO, Witlingo, and Brielle Nickoloff, Lead Product Marketing, Witlingo, discuss how voice will change the clinical research paradigm.

Bill Tobia, CEO of Clinstruct, Ahmed Bouzid, CEO, Witlingo, and Brielle Nickoloff, Lead Product Marketing, Witlingo, discuss how voice will change the clinical research paradigm.

Moe Alsumidaie sits down with Ping Yeh, CEO and co-founder of StemoniX, to discuss how MicroOrgans will transform preclinical and clinical development in challenging areas like Alzheimer’s.

Dr. Mark Smith, Chief Medical Officer of VistaGen Therapeutics, sits down with Moe Alsumidaie to discuss the challenges of modern psychiatric clinical trial design and implementation and their neuroactive pherine, PH48B.

Cancer therapy development has advanced to researching targeted immunotherapies and moving into gene-specific therapies. Some companies, however, are focusing on reviving cytotoxic therapies that were too toxic for patients when administered generally. Bill Newell, Chief Executive Officer of Sutro Biopharma, sits down with Moe Alsumidaie to discuss the use of a cell-free protein synthesis approach.

Nonadherence in clinical trials plays a significant role in influencing the quality of data, trial results and, subsequently trial cost and duration. It may stem from unintentional drivers, such as forgetfulness, poor organizational skills, protocol regimen complexity, or experiencing an Adverse Event.

Karen Hill and Heather Fitzpatrick Medlin discuss the recent advancements and challenges in cardiovascular and metabolic studies, as well as strategies to address current obstacles and advance the industry further.

Insights from ExL’s 5th Clinical Trials Phase I & Phase IIA Summit on early phase trials.

In this article, Moe Alsumidaie will discuss how my study teams manage monitoring reports and offer a tracking tool to assist sites with the process.

In this article, Moe Alsumidaie will identify several bottlenecks that study sites experience from the subject recruitment to the enrollment phase.

In this interview, Rashieda Gluck, Senior VP, Global Clinical Operations, at Aurinia Pharmaceuticals, will discuss how patient centricity techniques are benefiting smaller biopharmaceutical companies.

2018 has been an exciting year in the clinical trials industry, as we have seen many changes in novel concepts, and the evolution of some concepts to the point where initiatives and pilots are crystalizing into common practice.

In this article, Moe Alsumidaie will discuss QA updates.

In this interview, Carolyn Brehm, BMS Study Connect Business Lead, will discuss how BMS is helping to match patients with studies through Study Connect.

In this interview, Katie Mazuk, Global Head Investigator & Patient Engagement, will discuss her perspectives on improving patient connectivity.

Peter Benton, President and Chief Operating Officer of Worldwide Clinical Trials (Worldwide), will discuss areas of opportunity, CRO differentiation strategies, and organizational diversity.

At PanAgora’s Clinical Trials Patient Experience Summit, three main topics stood out; companies engrained patient centricity guiding principles in their operational models, the new concept of patient connectivity is emerging, and digital health is rapidly gaining ground in clinical trials.

In this interview, Lisa Dilworth, as VP, Rare and Orphan Diseases at Synteract, will discuss common challenges with rare disease studies, how to encounter them, and how the rare disease space is evolving.

In this interview, Michael J. Graziano, PhD, DABT, Vice-President, Drug Safety Evaluation for Bristol-Myers Squibb and leader of the BioCelerate Toxicology Data Sharing Initiative, provides more details about the DataCelerate platform.

This article will summarize the discussion on innovation that took place at this years Cambridge Health Institute’s Clinical Trial Innovation Summit.

In this article, Moe will elaborate on how to run an effective and engaging social media campaign on Facebook.

In this interview, Sudip Parikh, SVP and Managing Director of Americas at DIA Global, will discuss the latest industry trends, and how DIA NOW is helping to facilitate information across the clinical trials industry.

In this interview, Jim Streeter, Global Vice President of Life Sciences Product Strategy at Oracle, will elaborate further on Oracle’s strategy with mHealth in clinical trials.

In this interview, Linda Rawlings, VP of Neurodegenerative Development at Synteract, elaborates on addressing the challenges of neurodegenerative disease clinical trials.
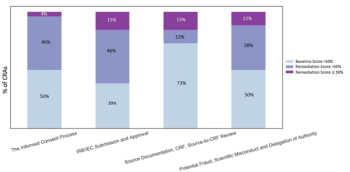
In this article, we will demonstrate a methodology that resulted in improving CRA performance.

In this article, we will discuss trends and challenges with medical monitoring clinical analytics, analyze the alignment of existing medical monitoring tools and technologies with ICH-E6 (R2) addendum guidelines, and discuss trends in medical monitoring insourcing and outsourcing models.
John Reites, THREAD’s Chief Product Officer, will discuss eDROs and the FocalView App in this interview.
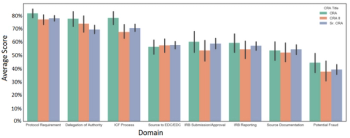
A global CRO’s data gathered using an objective monitoring simulation administered by CRA Assessments, LLC reveals that CRAs are consistently underperforming regardless of the level of experience or training.

The biopharmaceutical industry continues to explore how mHealth can change clinical trials, as the discussion continued at Hanson Wade’s mHealth for Clinical Trials EU Summit in London.

In this interview, Munther Baara, Head of New Clinical Paradigms at Pfizer, will discuss his perspectives on how blockchain may impact clinical trials.

Newest revelations suggest Risk Assessment Categorization Tools are now becoming therapeutic area-specific, as an oncology-based risk library is now surfacing.