Adzynma was previously granted a FDA Rare Pediatric Disease Priority Review Voucher, as well as Priority Review, Fast Track, and Orphan designations for patients with congenital thrombotic thrombocytopenic purpura.

Adzynma was previously granted a FDA Rare Pediatric Disease Priority Review Voucher, as well as Priority Review, Fast Track, and Orphan designations for patients with congenital thrombotic thrombocytopenic purpura.
The FDA granted accelerated approved in May 2021 to pembrolizumab (Keytruda) plus trastuzumab (Herceptin), fluoropyrimidine, and platinum-containing chemotherapy for the treatment of locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma.
Fruzaqla may help to provide a significant survival benefit to patients with metastatic colorectal cancer without negatively impacting quality of life.

Tirzepatide (Zepbound; Eli Lilly and Company) is as the first and only FDA-approved medication for obesity that activates GIP and GLP-1 hormone receptors.

Rexulti was found to be a well-tolerated treatment for the debilitating symptoms of agitation associated with dementia due to Alzheimer’s disease.
Pegloticase (Krystexxa; Amgen) is approved to treat chronic gout in adults who fail to normalize serum uric acid and whose signs and symptoms are inadequately controlled with xanthine oxidase inhibitors.

AI could present an innovative path forward in reshaping the landscape of global health strategies.

The newly approved Alinity m high risk human papillomavirus (HPV) assay is indicated to detect HPV and for use in routine cervical cancer screening per professional medical guidelines.

The FDA placed a partial hold on a phase 1 trial for the Bruton tyrosine kinase degrader NX-2127 for the treatment of patients with relapsed/refractory B-cell malignancies.

Phathom Pharmaceuticals announced that it anticipates vonoprazan (Voquenza) to be commercially available by December 2023.

The branded form of secukinumab is currently the only FDA-approved fully human biologic that directly inhibits interleukin-17A.

Abatacept is indicated across multiple inflammatory conditions, including for the treatment of adult patients with moderately to severely active rheumatoid arthritis, pediatric patients with moderately to severely active polyarticular juvenile idiopathic arthritis, and active juvenile psoriatic arthritis.

The FDA granted Wezlana with interchangeable designation after clinical trials found no clinically significant differences in safety and efficacy for the indicated conditions across multiple inflammatory diseases.

The approval of pembrolizumab (Keytruda; Merck) combined with gemcitabine and cisplatin for the treatment locally advanced unresectable or metastatic biliary tract cancer is the sixth sixth indication for the anti-PD-1 therapy for gastrointestinal cancers.

The two groups will implement AI technology with an already existing precision medicine trial model.

EMA reminds the clinical trials community that the Clinical Trials Information System will be mandatory at the last day of this month.

Sam Whitaker, co-founder and co-CEO of Mural Health discusses his time in the industry and patient needs in the current clinical trials market.
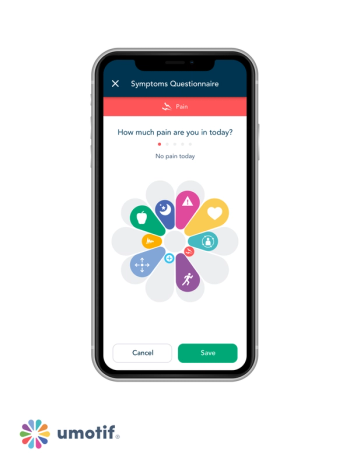
Study shows eCOA/ePRO interface is equivalent to responses from text-based smartphone formats.

Tufts CSDD report based on data collected and analyzed financial, personnel, and trend data from 18 top public and private CROs.

The Digital Medicine Society (DiMe) CEO Jennifer Goldsack and Takeda’s Head of Digital Health Sciences, Data Sciences Institute Shoibal Datta describe the current landscape of digitized clinical trials and the decisions needed to fully utilize their potential.

Andrea Zobel, PhD, Senior Director of Personalized Supply Chain at AmerisourceBergen��’s World Courier, and Graham Wylie, MBBS, Executive Chairman & Founder of the Medical Research Network (MRN), discuss the demand for DCTs, challenges surrounding them, and what their future holds.

Remote inspection and ICH guidelines among topics discussed at Veeva's most recent TMF Innovation Forum.

Rik de Greef, senior vice president of global quantitative science services at Certara discusses the organization's training initiatives in Africa.

Porter discusses regulatory compliance, ATMP sponsors, and Philadelphia’s Cellicon Valley.

Tony Clapsis, SVP, Clinical Trials Services, CVS, discusses recent advancements in the clinical trials space by CVS.

Darcy Flora, PhD, chief research officer of GRYT Health, a digital oncology company, discusses her career, the company, and drug development.

InterveXion CEO Keith Ward and Andrew Schafer, vice president of strategy for Biospatial discuss the details of a study which utilizes EMS data to identify sites for a Phase II clinical trial.

Elizabeth Rickenbacher, PhD, Director of Strategy for 4G Clinical discusses changes in the IRT and RTSM solutions.

Physicians share their experiences integrating research into practice.

Denis Polyanskiy, founder of Trialcome, discusses how the industry can overcome issues with non-adherence.