
Phase II open-label, non-comparative, multicenter clinical trial shows Cresemba is effective in pediatric patients as young as 1 year of age with invasive aspergillosis and invasive mucormycotic.

Phase II open-label, non-comparative, multicenter clinical trial shows Cresemba is effective in pediatric patients as young as 1 year of age with invasive aspergillosis and invasive mucormycotic.
Casgevy, which was approved along with Lyfgenia, is the first approval for a novel genome editing technology for sickle cell disease, which represents a significant advancement in gene therapy.
KEYLYNK-008 trial finds that Keytruda in combination with chemotherapy followed by Keytruda plus Lynparza did not produce an improved overall survival benefit in patients with metastatic squamous non-small cell lung cancer.

Clinical trials demonstrated superiority of Fabhalta to anti-C5s in hemoglobin improvement in the absence of transfusions and transfusion avoidance rate, showing clinically meaningful hemoglobin-level increases without the need for blood transfusions in patients with paroxysmal nocturnal hemoglobinuria.

Johnson & Johnson’s TAR-200 is currently in clinical trials for patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer who are ineligible for or elected not to receive bladder removal surgery.
Opdivo in combination with cisplatin-based chemotherapy shows durable responses and improved survival for patients with metastatic urothelial carcinoma.
Pirtobrutinib (Jaypirca) is a next-generation, highly selected, non-covalent BTK inhibitor that has shown nanomolar potency against wild-type and C481-mutant BTK in cell and enzyme assays.
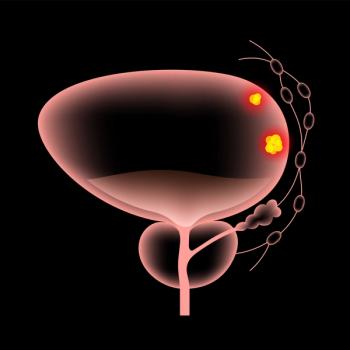
Supplemental Biologics License Application submission based on data from the Phase 3 KEYNOTE-A39 trial comparing Keytruda plus Padcev with chemotherapy of gemcitabine plus cisplatin or carboplatin.
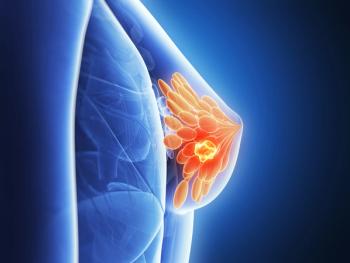
FDA grants to expedite review of zotatifin plus Faslodex (fulvestrant) and Verzenio (abemaciclib) for patients with ER–positive, HER2-negative advanced or metastatic breast cancer.
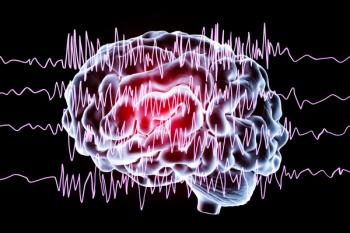
Included in the FDA warning were levetiracetam, under the brand names Keppra and Keppra XR; Elepsia XR; Spritam; and clobazam, under the brand names Onfi and Sympazan.

KRP203 (mocravimod) is intended to improve outcomes after hematopoietic stem cell transplantation for the treatment of hematologic malignancies.
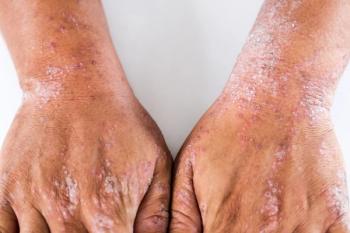
Arcutis Biotherapeutics’ supplemental new drug application was supported by significant findings from a trio of Phase 3 trials, a Phase 2 dose ranging trial, and a pair of Phase 1 pharmacokinetic studies for roflumilast cream 0.15% to treat atopic dermatitis in patients 6 years of age and older.

Data from the EMERGENT clinical trial program show Karuna Therapeutics, Inc’s KarXT (xanomeline-trospium) produced statistically significant and clinically meaningful improvements in the symptoms of schizophrenia.

The clearance includes the Vivos Therapeutics, Inc's DNA oral appliance, the mRNA oral appliance, and the mmRNA oral appliance for adults with severe obstructive sleep apnea.
The Elecsys HBeAg quant can detect the presence and quantity of the hepatitis B e antigen (HBeAg) in human serum and plasma.

Results from the Phase 3 DeFi trial (NCT03785964) showed that nirogacestat (Ogsiveo) reduced the risk of disease progression or mortality by 71% compared with placebo.
Epcoritamab-bysp (Epkinly; AbbVie and Genmab) is a T-cell engaging bispecific antibody under evaluation for the treatment of adults with follicular lymphoma that is relapsed or refractory following treatment with two or more therapies.

The FDA granted Fast Track Designation to ADP101 based on findings from the phase 1/2 Harmony trial (NCT04856865), which analyzed the safety and efficacy of the novel treatment in desensitizing patients with single or multiple food allergies.

First-in-class combination of capivasertib (Truqap) plus fulvestrant (Faslodex) approved for patients with HR-positive, HER2-negative locally advanced or metastatic breast cancer with one or more PIK3CA, AKT1 or PTEN biomarker alterations.
Astellas Pharma and Pfizer Inc's enzalutamide (Xtandi) gets FDA approval to treat nonmetastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastasis.
Merck’s pembrolizumab (Keytruda) combined with fluoropyrimidine- and platinum-containing chemotherapy approved for the first-line treatment of patients with locally advanced unresectable or metastatic, HER2-negative gastric or gastroesophageal junction adenocarcinoma.
The FDA's approval of Bristol Myers Squibb’s repotrectinib (Augtyro) was based on findings from the open-label, single-arm, Phase 1/2 trial TRIDENT-1, which evaluated the safety, tolerability, pharmacokinetics, and anti-tumor activity of the drug in patients with advanced solid tumors.

The Phase 3 LOCK-IT-100 clinical trial for DefenCath was recommended for an early termination based on the demonstrated efficacy in treating catheter-related bloodstream infections in adults with kidney failure administered chronic hemodialysis.

Patient and public involvement and engagement has been used for applied research projects, including clinical trials, but has been lacking in statistical methodology research.

In addition to its latest indication, PD-L1 IHC 22C3 pharmDx can help identify patients with non–small cell lung cancer, esophageal squamous cell carcinoma, cervical cancer, head and neck squamous cell carcinoma, and triple-negative breast cancer who may derive a benefit from treatment with pembrolizumab (Keytruda).

The immense volume of available chemical compounds creates a significant challenge in the drug discovery process.

Study highlights the potential of a comprehensive, multistakeholder-driven data analysis pipeline that addresses individual data sharing, core data set sharing, and federated model sharing.

FDA expands indication for Pacira BioSciences, Inc's bupivacaine liposome injectable suspension (Exparel) as an adductor canal block and a sciatic nerve block in the popliteal fossa.

A pivotal Phase 3 trial for Ixchiq showed a 98.9% seroresponse rate at 28 days following a single immunization to prevent disease caused by chikungunya virus.

Adalimumab-bwwd (Hadlima; Samsung Bioepis Co., Ltd. and Organon & Co.) could become the third Humira biosimilar deemed interchangeable, following Cyltezo and Abrilada.