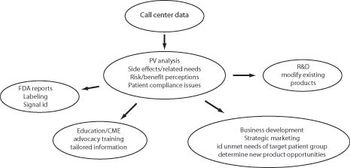
Help desk centers offer sponsors the chance to mine valuable PV data.

Help desk centers offer sponsors the chance to mine valuable PV data.

A look at the directive's flaws and the differing views across Europe about how to fix it.

FDA is modernizing systems to better access data on drug effects, utilization, and safety.

Drug evaluation criteria could soon include an intrusive health technology assessment.
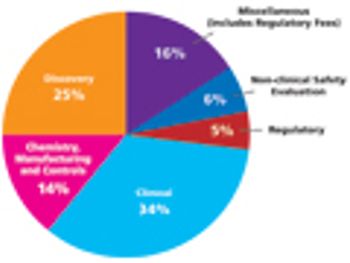
To successfully bring a drug to market and keep it there requires the skills of regulatory scientists.

New commissioner seeks to expand FDA's capabilities to bring safe and effective drugs to patients.

Through new pediatric networks, the EU hopes to improve clinical research in children.

Attention turns to its ederly population and their lack of participation in clinical research.



EMEA responds to more medicines, increase in complex procedures, and plethora of committees.

Globalization and reform initiatives will take part in shaping biomedical research and clinical studies.

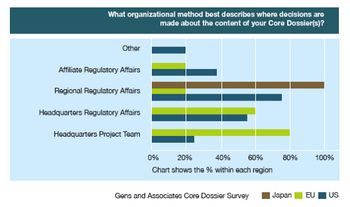

A comprehensive listing of U.S. departments and offices that includes the telephone numbers of directors, commissioners, and advisors.
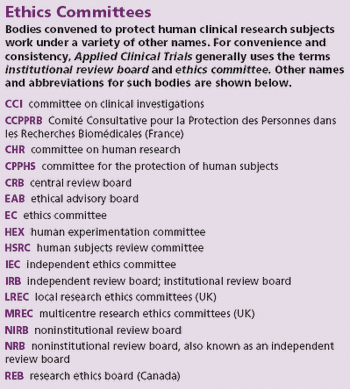
The latest version of the glossary, providing hundreds of definitions for key terminology related to clinical research.

Find out the latest on industry organizations and what they offer as membership benefits.

A listing of organizations, colleges, and universities that offer courses specific to advancing the education of clinical research professionals.

The management board has a supervisory role with general responsibility for budgetary and planning matters, the appointment of the Executive Director, and the monitoring of the Agency's performance.

Stay in the know with this list of up-to-date, commonly spoken, shortened words that are used often among clinical researchers.
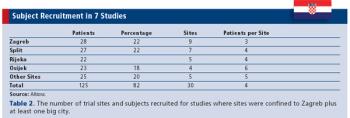
As a part of Europe, but not the European Union, trials in Croatia can encounter special challenges

Health concerns require EU regulators to become more involved in the politics of health care.

FDA reviewers strive to assess new postmarketing programs while also evaluating applications.
How Italy's new quality-driven regulations will ask more of CROs conducting activities in the country.

Why knowing a drug's risk and preparing for a risk evaluation mitigation strategy early on will considerably benefit a drug's development.