
News


To really transform global research we need to agree on a common language.

Industry news focusing on the people and organizations who work in the clinical trials profession.


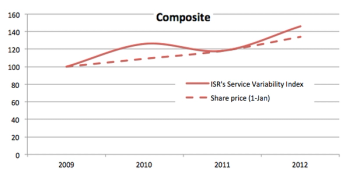
Quality is not just a way to gratify sponsors or authorities, but should be part an established and dynamic QMS.
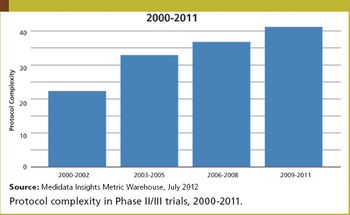
Medidata


Sites: Physician's Attitudes Toward Participation Also in this issue: Europe's Tentative New Rules Common Language for Global Research Quality Awareness in CROs



Video slideshow interview of Cynthia Hahn research compliance officer at Feinstein Institute for medical research

ImageIQ, a Cleveland Clinic-founded contract research organization providing imaging analysis software and services, announces that it has been chosen by Histogenics, a regenerative medicine company, to provide imaging analytics for the Phase III IND clinical trial of the company's NeoCart implant. NeoCart is an autologous bioengineered neocartilage grown outside the body using the patient's own cells for the regeneration of cartilage lesions.















