The Drug Information Association (DIA) is preparing to celebrate 25 years of training and education provision at its annual EuroMeeting.

The Drug Information Association (DIA) is preparing to celebrate 25 years of training and education provision at its annual EuroMeeting.

The end of the highly acrimonious election campaign has signaled a shift by policy-makers and interest groups to focus on dealing with the so-called government "fiscal cliff."
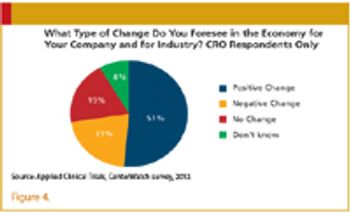
The rocky economy continues to wreak havoc on employment and drug development.