
New release further expands biologics capabilities and provides new tools for formulation scientists

New release further expands biologics capabilities and provides new tools for formulation scientists

Today, PSI CRO, a full-service contract research organization, announced opening of a new operational office in Italy.

Strengthens PPD?s comprehensive suite of feasibility, patient recruitment and retention services

An overview of the webcast covering the evolution of medical imaging in clinical trials, the current status of clinical trial imaging in oncology and its future directions


Registration of sites is start of an open, collaborative system for clinical research, connecting research sites worldwide through a shared technology platform.

I was lucky to be privy to early results from research being conducted on Risk-Based Monitoring.
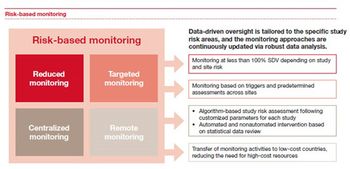
Although both the US Food and Drug Administration and the European Medicines Agency provided guidance on predictive analytics techniques in the development of a risk-based approach to the monitoring of clinical trials as far back as 2011, pharmaceutical and life science companies have been slow to fully adopt this approach across their study portfolios.

ICON plc, (NASDAQ: ICLR) a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries, today launched ICONIK Monitoring, the first in a series of new services that will leverage its ICONIK technology platform.

Huron Consulting Group (NASDAQ: HURN), a leading provider of business consulting services, today announced a strategic alliance with Forte Research Systems, a leading provider of enterprise research software, to enhance joint offerings to research institutions.

Virtify, Inc.,a content and regulatory information management solutions provider for the life science industry, today announced Indiana University has signed an enterprise-level contract for its Clinical Trial Registration & Results (CTRR) disclosure software.

Today, PSI CRO, a full-service contract research organization, announced their expansion to India and the appointment of Dr. Radhika Bobba as Regional Director for India and the Asia-Pacific region.

New legislation for transparency - currently underway for clinical trial data, national drug pricing rules, and personal privacy - is clearly not enough to satisfy the transparency hawks in Europe.

Woodley Equipment Company, a leading specialist medical and laboratory equipment supplier to the Clinical Trials industry has welcomed a new member to their Team.

When we hear about a new blockbuster drug coming to market, we usually think about a medical treatment.

A recent NY Times piece by Tara Parker-Pope reported on the recommendation by a group at the National Cancer Institute to change both the definition of cancer as well as treatment and detection techniques.

CROs utilizing more bonuses to attract and retain talent

Acquisition will further increase the depth of Certara?s early drug development analysis, modeling and simulation expertise

The Association of Clinical Research Professionals (ACRP) and the Alliance for Clinical Research Excellence and Safety (ACRES) have joined forces to address critical challenges facing the clinical research enterprise globally and to improve medical product development and delivery worldwide.

Theorem Clinical Research has announced the addition of RadMD, a cutting-edge medical imaging expertise company, to its roster of strategic alliances.

Enables secure global document sharing and community management tools for life sciences

CluePoints, a leading provider of Centralized Statistical Monitoring (CSM) solutions for clinical trials, has announced that its CSM techniques, that have been developed to improve data quality in studies, have been cited by the FDA in the recent release of the final guidance document detailing the agency's stance on the "Oversight of Clinical Investigations".

JAMA recently announced a change in editorial policy whereby it will no longer have independent academic statisticians review industry-sponsored clinical trial data.

New Service Enables Combination Tumor Assessments using both RECIST and irRC
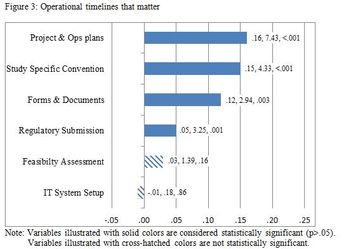
This study is grounded on the idea that in order to improve study startup performance, you must first be able to identify those key drivers of performance.

I'm spending some time going through the Draft Guidance for Industry Oversight--A Risk-Based Approach to Monitoring, August 2011 vs. the Guidance for Industry--Oversight of Clinical Investigations--A Risk-Based Approach to Monitoring, August 2013,

ICON plc, (NASDAQ: ICLR)a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries, and the National Clinical Trials and Research Centre (NCTRC) at theNational Taiwan University Hospital, today announced a collaboration agreement to enhance the set-up and management of clinical studies in Taiwan.

MedNet Solutions, a global life sciences technology company specializing in clinical study management systems, and Heart Imaging Technologies (Heart IT), an industry leader in web-based medical image viewing and management solutions, are pleased to announce their strategic partnership and the integration of Heart IT?s WebPAX technology with MedNet?s eClinical solutions.

In July, at a meeting called by the U.S. Department of Health and Human Services on Human Research Protections, I reviewed the fascinating results of a randomized study that recently compared paper-based informed consent to electronic informed consent.

We?re already embroiled in the annual speculation game about whether FDA approvals this year will keep pace with last year?s near-record of 39 new molecular entities (NMEs) brought to market.