News


Comprehensive support for trials of any size or complexity whether they originate in Asia or include Asian sites
Scientists and experts have conducted extensive research on protocol optimization and the need to enhance study efficiency, and sponsors are starting to look at their study design strategies.

Competitiveness in the field of industry-sponsored clinical research is largely contingent on speed, quality, and cost.

Integrating a CTMS and an EMR can provide many benefits for supporting the health care needs of patients.

For the past few decades, contract research organizations have successfully fulfilled a 'body-shop' role in clinical development...
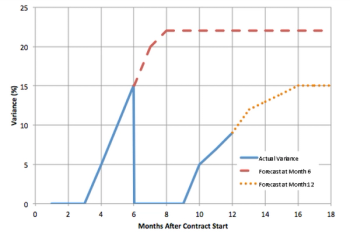
Let's look at a valuable cost metric: percent variance from budget.

Bangalore and North Carolina facilities look to expand staff after the award of two large FSPs

Quanticate is expanding operations in both its Bangalore, India and Research Triangle Park, NC locations.

Acquisition provides customers improved access to Middle East and North Africa region as well as Southern Europe

Acquisition provides customers improved access to Middle East and North Africa region as well as Southern Europe
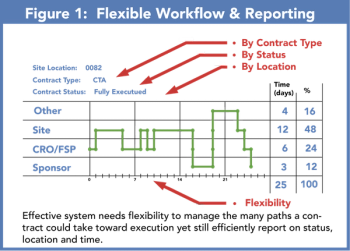
The management and oversight of clinical trials agreement contracts are still handled through spreadsheets or document management systems ill equipped for an efficient CTA lifecycle.

KCR has opened new offices in London and Berlin, and is now turning its attention to France.

Despite a slight boost in funding for the Food and Drug Administration and stronger tax incentives for investment in R&D, significant changes in Medicare drug reimbursement and coverage policies have biopharmaceutical companies up in arms.

Celerion Opens Operations in South Korea at Seoul National University Hospital Clinical Trial Center
Celerion is pleased to announce the expansion of clinical operations to South Korea.

Celerion Opens Operations in South Korea at Seoul National University Hospital Clinical Trial Center
Celerion will expand clinical operations to South Korea.

Expanded membership and new process to prequalify providers among 2014 priorities.

The Avoca Quality Consortium announced its key initiatives for 2014.

DIA Exhibitor Form 2014

Proteomics International Partners with inVentiv Health Clinical

EMA's Executive Director, Guido Rasi received the 2014 European Rare Disease Leadership Award 2014.

Partnership offers developers full service biosimilar expertise on a global scale

Dennis Gillings, PhD, Executive Chairman of Quintiles, has been appointed the first world dementia envoy.

Novartis has renewed its commitment to clinical trial data transparency

Experienced leaders form Provision Research Compliance Services, a joint venture between Schulman Associates IRB, Inc. and Falcon Consulting Group, LLC
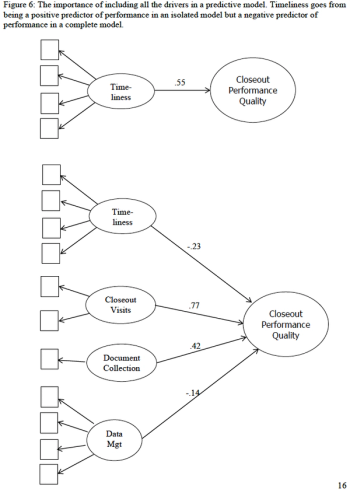
Organizations involved in clinical trials have embraced the idea that they should collect performance measures to assess how well they conduct clinical trials.

...a panel discussion was held with CEOs of top generic pharmaceutical companies and key financial analysts.

Would you delay protocol approval by two months if it meant your enrollment and retention would improve and, in turn, positively affect your data quality?

A panel discussion was held with CEOs of top generic pharmaceutical companies and key financial analysts...

Schulman Associates IRB, Inc. and Falcon Consulting Group, LLC announced they have formed a joint venture