
News


Double-blind randomized controlled trials of new drugs may fail to measure how a medication’s performance can vary based on patients' lifestyle choices, especially if patients change their habits because they are anticipating treatment, according to a new study published in Plos One.



Comprehensive behavioral and performance data on over 40,000 investigator sites

Participate sets a new standard for usability, integration, and cost effectiveness for electronic patient reported outcomes (ePRO).


PatientsLikeMe and the FDA have signed a research collaboration agreement to determine how patient-reported data can give new insights into drug safety.

UK-based Medical Research Network (MRN) has entered into a strategic partnership with World Courier to create a more highly integrated model for community-based clinical trials.

In the U.S. alone, around $28 billion a year is spent on preclinical research that is not reproducible, and low reproducibility rates contribute to both delays and costs of therapeutic drug development.


The Society for Clinical Research Sites (SCRS), a global trade organization dedicated to the interests of clinical research sites, announced a two-year partnership with Bristol-Myers Squibb.

INC Research formally launched the first Site Advocacy Group (SAG), initially focused on central nervous system (CNS) protocols.


The Society for Clinical Research Sites (SCRS), a global trade organization representing the interests of clinical research sites, announced a two-year relationship with Center Point Clinical Services.

Challenging. Time-consuming. Expensive. Do words like these spring to mind when you contemplate the patient recruitment process for your clinical trials?


The congress will be held at the Congress Center Hamburg (CCH) in Germany on 6 to 8 April 2016. For the first time, it will feature one or more sessions on medical writing.

CROMSOURCE announces the launch of TalentSource Life Sciences, a new name and brand identity for its established staffing solutions service for the clinical life sciences industry.



The biosensor is a disposable, wireless patch worn on the chest. Its thin, light form factor is comfortable and discreet while providing healthcare professionals access to unprecedented amounts of continuous vital sign data on their patients.

Two leading drug development and regulatory consultancies announced they have formed a new strategic alliance to help life science companies navigate an increasingly complex global environment.
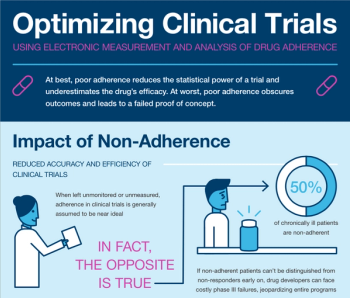
Drug development has never before been so difficult, time consuming and expensive. Accuracy in clinical trials, therefore, is a priority

Some can't miss sessions for the upcoming meeting.

Study Connect™ App Enables the Mobile Management of Randomization, Inventory Management and Unblinding in Clinical Trials

![AdvanstarMWV[Carrie]6-3-15-New-1433433049408.jpg](/logo.webp)
