
A look at the findings from Avoca Group’s 2018 industry research survey on clinical outsourcing.

A look at the findings from Avoca Group’s 2018 industry research survey on clinical outsourcing.

A strongly-worded plan for reining-in profit-driven drug firms receives immediate backlash as arguments over drug pricing and patient access continue to rage around Europe and the US.



A recent survey shows that 40% of pediatric trials conducted between 2008 and 2011 were never finished or published. One of the main challenges is the need to collect multiple sequential blood samples. Which, mircosampling, could be the answer to.



CEO of Synteract, Steve Powell, discusses the advantages that mid-sized CROs-like his own-offer emerging drug developers to navigate continually changing processes.



A strongly-worded plan for reining-in profit-driven drug firms receives immediate backlash as arguments over drug pricing and patient access continue to rage around Europe and the US.



COO and CMO of BrainStorm, Ralph Kern, discusses how his company is overcoming challenges and advancing science in neurodegenerative disease.



Chinese biotech companies are emerging as serious competitors in the global market as the country's drug development landscape claims industry attention once more.

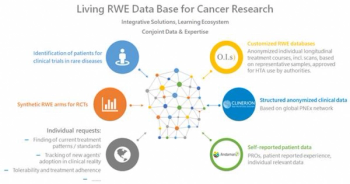









The industry-wide shift towards RBx is not only reducing risk, but improving oversight and boosting proactive, predictive decision making, writes Cluepoints' CCO, Patrick Hughes.

