
Applied Clinical Trials
Will ethics committees buy into the radical new approach of latest draft regulation?

Applied Clinical Trials
Will ethics committees buy into the radical new approach of latest draft regulation?


Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.


Applied Clinical Trials
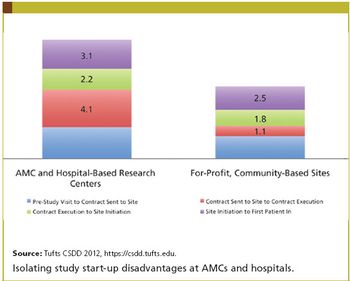
A framework is needed to facilitate, promote, and reward basic and applied research for adults and children.

Applied Clinical Trials
A countdown of the top 10 developments that have most influenced the ePRO industry over the past decade.
Applied Clinical Trials
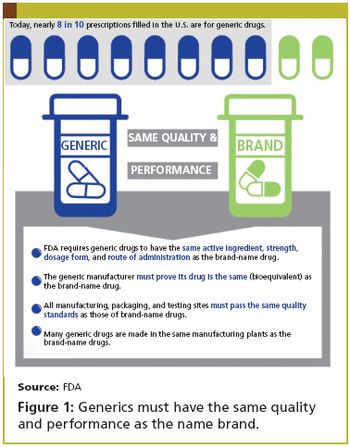
In late August, the FDA elevated the Office of Generic Drugs to report directly to Janet Woodcock, the director of CDER.
Applied Clinical Trials
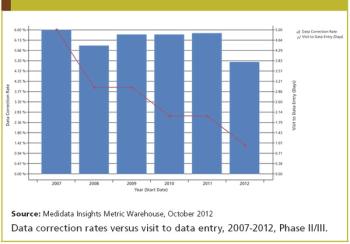
Medidata
Applied Clinical Trials
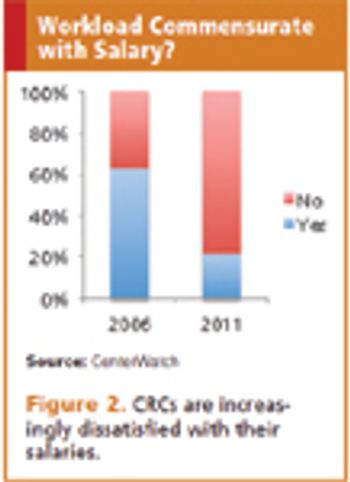
Study coordinators see responsibilities increase dramatically while salary levels remain flat.

Applied Clinical Trials
A mid-year summary of the European Medicines Agency's 2012 activities underlines the growing trend toward closer collaboration between international regulators.

Applied Clinical Trials
Recommendations for assessing translatability.

Applied Clinical Trials
Meeting FDA requirements: the electronic collection of suicidal ideation and behavior data.

Applied Clinical Trials
The key to accelerating drug development, according to numerous experts, is to revise requirements that generate lengthy, complex, and costly clinical trials.

Applied Clinical Trials
The opportunities and challenges social media provides in the realm of subject recruitment.

Applied Clinical Trials
Subject Recruitment: Recruitment Through the Use of Social Media Also in this issue: EU Regulation Protocol Study Coordinator Survey Development of Pediatric Drugs