
Applied Clinical Trials
Vienna joins growing list of bidders to land Europe's drug regulator; Amsterdam the early favorite?

Applied Clinical Trials
Vienna joins growing list of bidders to land Europe's drug regulator; Amsterdam the early favorite?

Applied Clinical Trials
New legislation aims to expand regulatory acceptance of patient data from healthcare systems and observational studies.

Applied Clinical Trials
New safety review of epilespy treatment puts spotlight on issues such as communication between regulators and prescribers.
Applied Clinical Trials
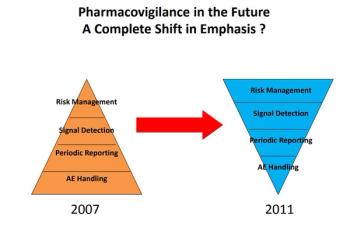
PV experts face new challenges as focus in field moves toward acting on real-world evidence insights.

Applied Clinical Trials
Q&A explores the evolution of the patient-centric supply chain and how it's poised to impact the clinical trials landscape.

Applied Clinical Trials
Recent study, and others in literature, inform misconceptions around physician and nurse involvement in clinical trials.
Applied Clinical Trials
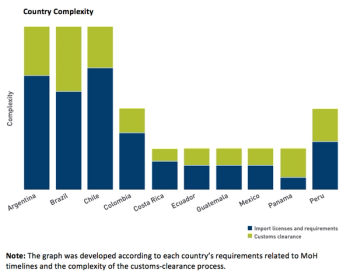
Supply chain strategies require a close look at the regulatory factors and the drug import hurdles and hopes in the region.

Applied Clinical Trials
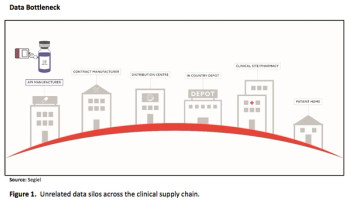
Automation will be key to improving efficiency and mitigating risk during the complex process of cell therapy production and delivery.
Applied Clinical Trials
How a single-source temperature management strategy can support a drug’s quality and integrity in transit-a process as important as the destination.

Applied Clinical Trials
Outlining a decision-making framework that integrates real-world signals with supply planning techniques to reduce risk and avoid potential interruptions.

Applied Clinical Trials
Learn to partner effectively in the era of collaboration, where the benefits gained will outweigh the challenges if approached correctly

Applied Clinical Trials
The key to moving legacy systems-electronic or paper-to the cloud: Do it in doses-and learn from those who went before.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials September/October 2017 issue in an interactive PDF format.