
Applied Clinical Trials
The need to revise the design, performance and interpretation of clinical research to reflect changing methods and standards is drawing increased attention.

Applied Clinical Trials
The need to revise the design, performance and interpretation of clinical research to reflect changing methods and standards is drawing increased attention.

Applied Clinical Trials
The uncertainty surrounding where the agency and its 890-member staff will call home next may linger for months, if not years.

Applied Clinical Trials
A look at the EMA’s proposed guidance revisions for first-in-human studies.

Applied Clinical Trials
Janssen releases key findings from its pilot study evaluating the use of electronic informed consent technology in clinical trials.

Applied Clinical Trials
A patient-centric approach to a Phase IV trial for an MS treatment resulted in lessons that can be applied to future therapeutic studies for this and other rare diseases.

Applied Clinical Trials
More precision models in connecting patients to clinical trials and treatment options are vying to close the ever-elusive recruitment gap.

Applied Clinical Trials
Survey delves deep into what constitutes true game-changing innovation in clinical development.

Applied Clinical Trials
The need to move beyond a point-solutions approach to one built around applications designed to manage the end-to-end clinical trial process is crucial.

Applied Clinical Trials
A proposed operational framework to support digital- and mHealth-based clinical trials in Africa.

Applied Clinical Trials
Exploring the benefits from cross-company data sharing for study planning and investigator selection.
Applied Clinical Trials
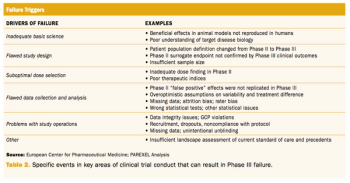
An examination of recent failures in Phase III studies and innovative approaches to reduce risk.

Applied Clinical Trials
Research reveals a need for sponsors to be much more consistent, disciplined and focused in their CRO usage.

Applied Clinical Trials
Study measures the differing judgment levels between clinical investigators and drug safety experts.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials August/September 2016 issue in an interactive PDF format.