Applied Clinical Trials
Survey uncovers the challenges, myths, and potential useful strategies associated with BYOD adoption.

Applied Clinical Trials
Survey uncovers the challenges, myths, and potential useful strategies associated with BYOD adoption.

Applied Clinical Trials
FDA's breakthrough drug initiative has proven successful to date, but challenges remain in addressing the expectations and patient concerns surrounding candidates in this program.

Applied Clinical Trials
Renewed attention on ways to tackle this age-old scourge is building in Europe and beyond.

Applied Clinical Trials
European forum tackles the problem of limited elderly patients involved in clinical trials.
Applied Clinical Trials
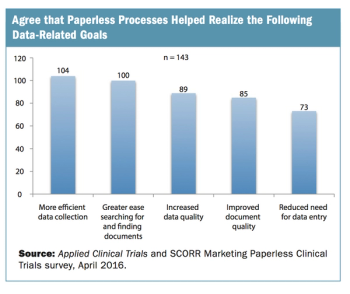
Survey reveals mixed adoption of paperless data collection in clinical trials, pointing to the need for greater alignment of new eClinical technologies and study conduct.

Applied Clinical Trials
Recent FDA draft guidance pushes for the use of electronic health record data in clinical investigations-and synching EHRs with research systems.

Applied Clinical Trials
CDER official comments on the significance of ICH's new alternative path for identifying the cardiac safety issue of QT prolongation in non-cardiac drugs.

Applied Clinical Trials
The need to involve regulators is crucial when the use of electronic data devices impacts the management of patient safety and evaluation of trial endpoints.
Applied Clinical Trials
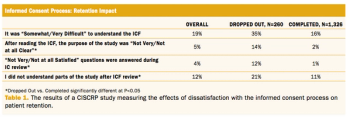
Why electronic informed consent is key to supporting today’s patient-centric mantra in clinical trials.

Applied Clinical Trials
Work burden and performance hurt by technology incompatibility.
Applied Clinical Trials
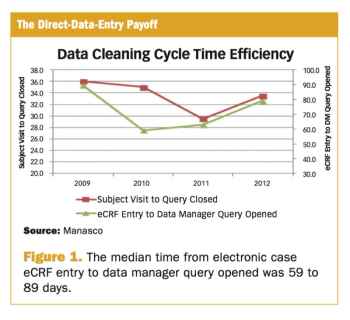
Outlining those technologies best able to raise the data and process quality of risk-based monitoring.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials June/July 2016 issue in an interactive PDF format.