
Applied Clinical Trials
Recent legislative and regulatory changes increase the country's appeal as a destination for research.

Applied Clinical Trials
Recent legislative and regulatory changes increase the country's appeal as a destination for research.

Applied Clinical Trials
Advancing medicine and the clinical research process with the help of patient organizations

Applied Clinical Trials
Reformers seek disclosure of investigator payments and conflicts of interest.
Applied Clinical Trials
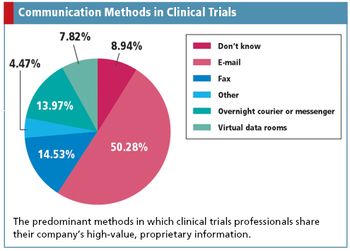
Uncovering the risky communication methods of clinical trials professionals, while discovering a potential Web-based replacement.

Applied Clinical Trials
London conference underscores foggy climate surrounding clinicaltrials.gov

Applied Clinical Trials
Drug development, largely immune to past economic downturns, now faces a different climate.

Applied Clinical Trials
On the scene with electronic content management.
Applied Clinical Trials
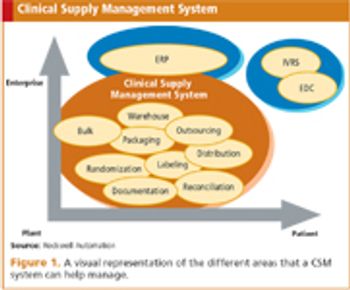
Improving the quality and speed of drug development through integration and interoperability.

Applied Clinical Trials
A clinical trials professional with personal experience as a trial subject discusses the purpose and importance of following protocol.

Applied Clinical Trials
Overcoming hurdles in subject enrollment by managing potential risks in six critical key areas.

Applied Clinical Trials
Ten-year-old study sets precedent for future collaborative observational studies on drug safety.

Applied Clinical Trials
The latest eclinical software in the clinical trials industry.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.