Applied Clinical Trials
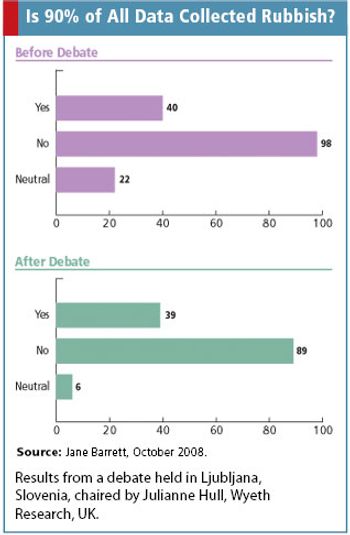
Jane Barrett discusses data quality as she addresses the question raised by DIA's 2nd European Clinical Forum: Is 90% of data rubbish?

Applied Clinical Trials
Jane Barrett discusses data quality as she addresses the question raised by DIA's 2nd European Clinical Forum: Is 90% of data rubbish?

Applied Clinical Trials
What antitrust authorities have uncovered so far in their search for sabotage.

Applied Clinical Trials
The latest eClinical software in the clinical trials industry.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.
Applied Clinical Trials
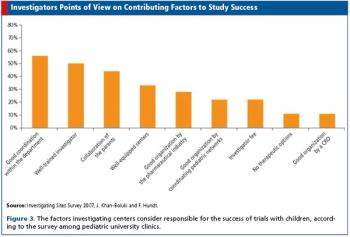
Surveys among pharma, clinics, and investigators shed light on trials in children.

Applied Clinical Trials
With greater attention and acceptance of biomarker use in clinical trials, the Society of Nuclear Medicine (SNM) is hoping to capitalize on its expertise in this area through the creation of the SNM Clinical Trials Network.

Applied Clinical Trials
A look at the vague but time consuming requirements imposed on sites and efforts to ease the burden.

Applied Clinical Trials
The case for standardizing the use of clinician rated outcome measures to improve research studies.

Applied Clinical Trials
A look into the current state of the eCTD format as well as its future in industry from a global standpoint.

Applied Clinical Trials
More resources and new leadership could help FDA regain its stature and bolster support for innovation.

Applied Clinical Trials
As patient recruiters and sponsors face low recruitment numbers, they must look beyond global trials to find a solution.

Applied Clinical Trials
Regional recruitment managers are on the rise, providing a unique approach to onsite subject enrollment.