
Applied Clinical Trials
This new model standardizes data exchange between the various stages of a research study.

Applied Clinical Trials
This new model standardizes data exchange between the various stages of a research study.

Applied Clinical Trials
Closing Thoughts on the state of clinical trials in India today.


Applied Clinical Trials
Lack of adequate and attentive support is preventing some sites from fully taking advantage of EDC and ePRO technologies.
Applied Clinical Trials
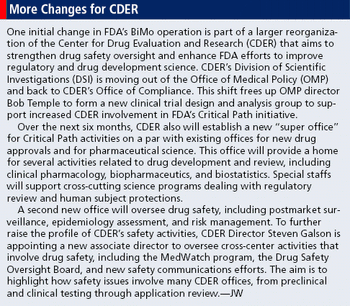
Changes prompt new oversight approaches by FDA and research institutions.

Applied Clinical Trials
As some EU countries struggle with the Directive's regulatory demands, emerging markets such as China, India, and Russia continue to bolster their clinical research and drug development programs.

Applied Clinical Trials
GRPs are not another set of guidelines or "additional hurdles" prepared by authorities.

Applied Clinical Trials
New paper from the European Medicines Agency explores future vaccine guideline.

Applied Clinical Trials
Prof. Marion E.T. McMurdo elaborates on the importance of including older subjects in clinical trials.