
Europe's clinical trial community has a lot to say about the EMEA's draft guidance on first studies in man.

Europe's clinical trial community has a lot to say about the EMEA's draft guidance on first studies in man.

Industry and patient groups embrace new rules but acknowledge concern over initiative's impact across EU.

After a long and winding journey, now the real work begins on the EU Regulation for all those involved.

New to the trials scene, many up-and-coming European firms are seeking the expertise of CROs they can trust.

Group recommends ways to improve and increase clinical research in new report.

Lack of regulatory cohesion across member states a source of frustration for many in industry.

There's more than meets the eye to the EU's new public-centric initiative to simplify drug safety.

Regulation will benefit both children & drug development, says EMEA, which has a lot on its plate this year.

UK report on last year's TGN 1412 trial provides industry with blueprint for improved early clinical studies.

Patient safety, orphan medicines featured in EU's six-year research support budget.

There's plenty to tackle this month, including a debate over efficacy, new ARDS guidelines, and evidence the EMEA is listening.

A new report from the European Union grades member states on their orphan drug incentives.

EMEA gives EU six months to respond to highly technical draft guidance on viral safety of biotech meds.
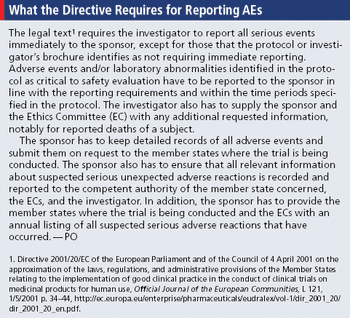
EU helps ease ambiguity of reporting process with recent published guidance.

European Medicines Agency adopts new approach to drug development process.

New Q&A guidance attempts to clear up any confusion about new Directive rules.

EU Council supports pediatric medicines proposal, but adds some changes.

Biomarkers take center stage in Europe, where their critical role is under examination.

The European pharma industry struggles to regain its former prowess in R&D.

As patents expire, clinical trials will be caught in the middle between copyists and original innovators.

New paper from the European Medicines Agency explores future vaccine guideline.

Political and technical tensions in the EU.

Three recent initiatives address the reputation of the health care industry in the EU.

Parliament's first review of the new pediatric rules stirs controversy.

The EMEA's guidance is a roadmap for understanding current and future guidelines.

Years of debate about pediatric research in the EU may finally end up going somewhere.

For the EU, these new good clinical practice rules come not from Brussels, but Mt. Sinai.

A recent conference featured debate on the role of research ethics committees.

Will the new pediatric and ethics initiatives receive enough funding to make them truly work?

Industry?s proposal for a shared database has merit, but is it ?too little too late??