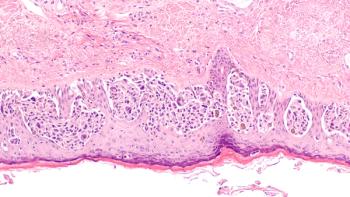
Results from a followup evaluation of COLOFOL trial show no significant improvement in 10-year overall or colorectal cancer–specific mortality rates between high-frequency and low-frequency postoperative follow-up regimens for patients with stage II or III colorectal cancer.