
Understand how intelligent study design, focused data collection, and faster analytics can help clinical teams deliver meaningful results that sustain investor momentum.

Understand how intelligent study design, focused data collection, and faster analytics can help clinical teams deliver meaningful results that sustain investor momentum.

Eli Lilly’s oral GLP-1 therapy, orforglipron, met all primary and key secondary endpoints in the Phase III ACHIEVE-2 and ACHIEVE-5 studies, showing significant A1C reduction, weight loss, and cardiovascular benefits compared to both dapagliflozin and placebo.

Gain insight into why early toxicology readiness and strong scientific collaboration with CRO partners are critical to accelerating trial startup and regulatory approval.

Uncover how improving data accuracy and leveraging synthetic control arms can optimize trial efficiency, reduce costs, and generate stronger real-world insights.

Examine strategies for validating, monitoring, and safely deploying configurable AI agents to ensure compliance and performance in clinical trials.

Capturing insights from clinical research professionals on the key trends and challenges shaping drug development today, from those in clinical trial operations and site relationships, to technology and AI, and the evolving regulatory and policy terrain.

Last week’s top stories explored how NIH’s shutdown plan is testing research resilience, why data infrastructure must precede AI adoption in 2025, and how sponsors are redefining outsourcing with hybrid resourcing models focused on flexibility, quality, and collaboration.

Take a closer look at how agentic AI can automate repetitive monitoring tasks while keeping human oversight central to critical decision-making in clinical research.

In this episode of the ACT Podcast, we highlight a recent Q&A featuring Ibrahim Kamstrup-Akkaoui, vice president of data systems innovation at Novo Nordisk; and a feature article by Chris Driver, senior director of product management, Patient Suite at IQVIA, in which they both highlight how sponsors are adopting automation to streamline operations.

Gain perspective on how agentic AI can bridge eCOA, EDC, IRT, and CTMS platforms to reduce manual effort and improve operational efficiency.

Understand how adaptive human-in-the-loop frameworks can maintain safety and decision quality as AI becomes more embedded in trial monitoring and data review.

Learn how streamlined confidentiality agreements and consistent workflows can speed site activation and improve sponsor-site transparency.

Gain insight into how listening to site feedback and prioritizing engagement, training, and local patient understanding can drive smoother startups and stronger study outcomes.

Gain insight into how AI-powered agents can eliminate inefficiencies, shorten development timelines, and free clinical teams to focus on strategic decision-making.

Explore ways to reduce redundancy in site training by applying adult learning principles and focusing on enrollment and randomization essentials.

The latest federal shutdown leaves the NIH operating with just one-quarter of its staff to maintain patient care at its Clinical Center, while broader funding cuts and proposed agency consolidations threaten the future stability of US biomedical research.

Discover how early site involvement, streamlined training, and AI-driven tools can simplify system complexity and enhance efficiency in clinical trials.

A look back at last week’s most-viewed content highlights how AI-driven monitoring could improve management of cytokine release syndrome in oncology, how biotech startups are leveraging scalable platforms to accelerate clinical trial operations, and why interoperability challenges remain a top concern for research sites.

Learn how internal infrastructure, cultural buy-in, and workflow-focused technology choices can strengthen collaboration and reduce site burden in clinical trials.

Learn how integrated platforms, single sign-on tools, and streamlined training approaches can reduce site burden while supporting compliance and study startup.

Discover how reducing redundancy in feasibility processes and leveraging shared databases can improve collaboration and ease site burden.

At the 2025 SCRS Global Site Solutions Summit, site leaders shared insights on when and how to sell a clinical research site, stressing the importance of timing, cultural alignment, personal goals, and clear terms for long-term success.

In a breakout session at the 2025 SCRS Global Site Solutions Summit, industry leaders from SCRS, QCR, and Syneos Health discussed how sites can prioritize patient empowerment, strengthen community outreach, and collaborate with CROs and sponsors to secure resources for engagement initiatives.

Learn how the lack of integration across platforms creates complexity for sites and why holistic solutions are key to reducing data entry and system burden.

At the 2025 SCRS Global Site Solutions Summit, industry experts discussed how sites can balance culture, staffing, and training while expanding across multiple locations.

Explore how stronger collaboration and closed feedback loops can improve communication, change management, and site engagement in clinical trials.

Steve Rosenberg, CEO, uMotif, discusses the challenges sites face with eCOA, eConsent, and ePRO platforms and the support needed to reduce technical burdens.

Last week’s top stories explored the push for digital-first clinical data systems, why cybersecurity must be central in CRO qualification, and how AI-driven contracting tools are helping sponsors cut delays and accelerate trial timelines.
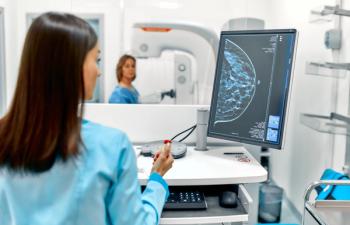
The FDA has approved Eli Lilly’s Inluriyo (imlunestrant), the first oral estrogen receptor antagonist for adults with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer, based on Phase III EMBER-3 results showing a 38% reduction in risk of progression or death versus endocrine therapy.

In this video interview, Ananth Kadambi, VP of real-world evidence and modeling solutions at Certara, highlights how clinical operations teams must enhance patient tracking, engage data monitoring committees earlier, and plan subgroup analyses to meet FDA’s overall survival guidance.