
Applied Clinical Trials
A changing research enterprise requires clearer policies and more effective regulatory tactics.

Applied Clinical Trials
A changing research enterprise requires clearer policies and more effective regulatory tactics.
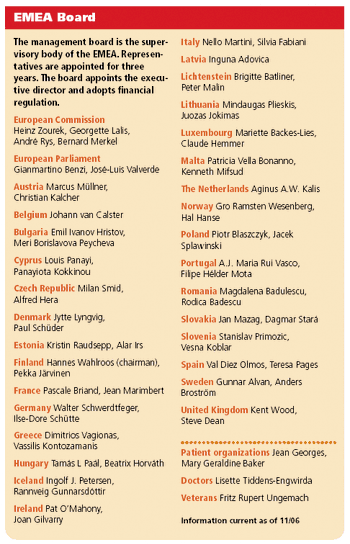
Applied Clinical Trials
Along with the titles and phone numbers of personnel, View from Brussels columnist Peter O'Donnell gives an update on the regulatory front in Europe.

Applied Clinical Trials
This newly updated glossary includes even more terms relevant to clinical trials professionals.

Applied Clinical Trials
Generalizing fastest drug development strategies and practices.

Applied Clinical Trials
This document from HHS and OHRP provides IRBs with instructions for reviewing trial information listed on Websites.

Applied Clinical Trials
Washington editor Jill Wechsler provides an updated list of U.S. regulatory agency personnel.

Applied Clinical Trials
These translations help make sense of initials commonly used by clinical researchers around the world.

Applied Clinical Trials
Today's technology narrows the gap between Web and desktop applications, transforming the user experience.

Applied Clinical Trials
Java applet and Adobe Flash are two of today’s technologies that come close to replicating the dynamic and highly functional environment of desktop software on the Web.