
Applied Clinical Trials
Pharmacists and patient groups welcome the new European Union directive on pharmacovigilance.

Applied Clinical Trials
Pharmacists and patient groups welcome the new European Union directive on pharmacovigilance.
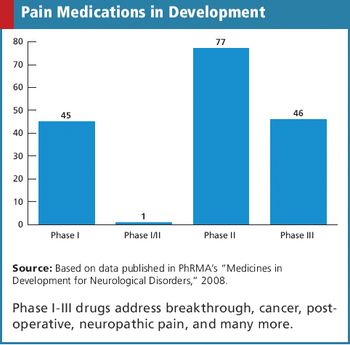
Applied Clinical Trials
Collecting too many metrics can lead to misappropriation and misinterpretation.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.


Applied Clinical Trials
Routine inspections and regulations can help maintain GCP standards in global trials.

Applied Clinical Trials
Reform law requires tracking and disclosure of fees to investigators and research consultants.

Applied Clinical Trials
Access to all available medical evidence can profoundly improve treatment effectiveness.
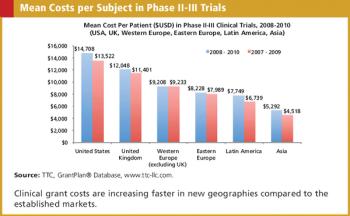
Applied Clinical Trials
Clinical grant expenditures represent a major portion of the budget for later phase clinical trials.

Applied Clinical Trials
Waiving inclusion/exclusion criteria affects investigators, subjects, sponsors, and the trial itself.

Applied Clinical Trials
The act creates serious concerns about the industry's ability to recruit and retain well-qualified investigators.
Applied Clinical Trials
Knowing when to outsource and when to conduct pharmacovigilance in-house is a crucial decision.