Applied Clinical Trials
The latest eClinical software in the clinical trials industry.

Applied Clinical Trials
The latest eClinical software in the clinical trials industry.

Applied Clinical Trials
How the Internet is blurring the lines between patient and physician and redefining old roles.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
Insight into obtaining insurance for a clinical trial and if or when it should be left to the professionals.

Applied Clinical Trials
The EU provides extensive funds to the clinical trials industry in an effort to better health care delivery.

Applied Clinical Trials
An in depth look at industry's reaction to FDAAA, its requirements, submitting the results, and meeting the deadline.

Applied Clinical Trials
From pre- to postmarketing, safety monitoring must be a priority that's handled with knowledgeable care.

Applied Clinical Trials
A purely empirical approach to drug development may allow for evaluation of the overt factors that impact the balance of benefit and risk, but it will never uncover all potential scenarios for which companies are now being held accountable.

Applied Clinical Trials
By defining hurdles to registration, emerging companies benefit from early regulatory guidance.
Applied Clinical Trials
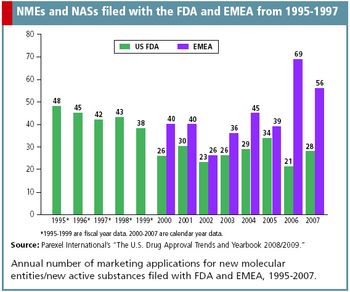
After almost a decade of dim progress in drug development, R&D is finally making a come back, according to current data published in Parexel International's, "The U.S. Drug Approval Trends and Yearbook 2008/2009."

Applied Clinical Trials
The Netherlands has become the latest European country to establish a network that is designed to promote pediatric drug development.