
Applied Clinical Trials
To realize the goal of the directive, member states need to collaborate and make improvements.

Applied Clinical Trials
To realize the goal of the directive, member states need to collaborate and make improvements.

Applied Clinical Trials
Instructions for evaluating adverse event data and guidelines for taking the proper course of action

Applied Clinical Trials
Regulatory submission solution addresses the needs of smaller biotech and pharma companies

Applied Clinical Trials
Phase I package adds formulary accountability, recruitment, lab, and EDC features

Applied Clinical Trials
There are a number of factors potential subjects need to consider when deciding whether to participate in a clinical trial, including an often overlooked matter that this month's authors examine: taxes.

Applied Clinical Trials
System enables IRB members to review ?s in near real-time from anywhere in the world

Applied Clinical Trials
Confusion over policies governing prisoners points to the need for clear, uniform safeguards for all research.
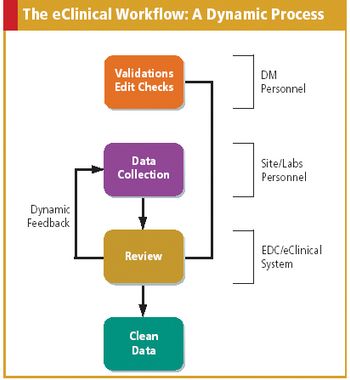
Applied Clinical Trials
As more companies adopt eClinical technologies, data managers must redefine and update their role.

Applied Clinical Trials
The importance of human subject protection, clear communication, and community spirit are among the issues emerging from the tragedy.

Applied Clinical Trials
An insightful review of Remedica's 2005 Clinical Trials: A Practical Guide to Design, Analysis, and Reporting
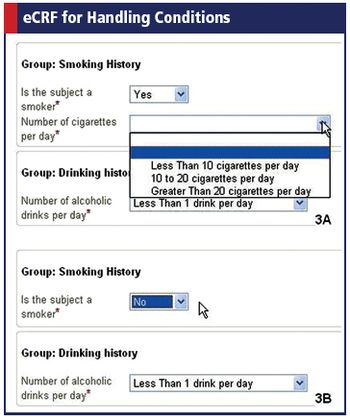
Applied Clinical Trials
The group's latest ODM release strengthens global reach and lets researchers define conditions in eCRFs.
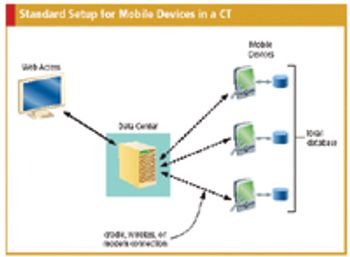
Applied Clinical Trials
Practical guidance for effectively integrating PDAs and other handheld devices into CTs

Applied Clinical Trials
A new report from the European Union grades member states on their orphan drug incentives.