
Applied Clinical Trials
The EMA is committed not just to greater accessibility of data, but proactive publication.

Applied Clinical Trials
The EMA is committed not just to greater accessibility of data, but proactive publication.
Applied Clinical Trials
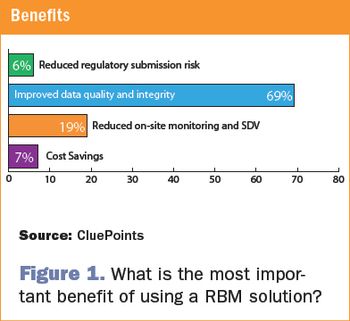
Central statistical monitoring can improve RBM solutions by efficiently detecting errors, sloppiness, tampering, and fraud.

Applied Clinical Trials
Everyone is looking to rev up the biopharmaceutical development pipeline, and the FDA is working hard to do its part.
Applied Clinical Trials
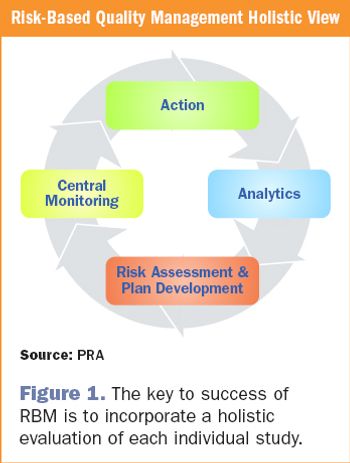
The reality of risk-based monitoring: history and successful implementation for late phase research.

Applied Clinical Trials
The implementation of best practices for clinical study and development conduct can streamline administrative burdens for investigator staff as well as study teams, and hopefully yield reduced costs in conducting global clinical development.

Applied Clinical Trials
Are clinical trial data shared sufficiently today?
Applied Clinical Trials
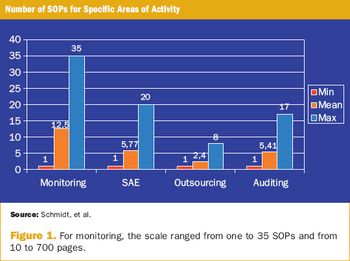
Survey appraises the use of SOPs in clinical research from the sponsors' point of view.

Applied Clinical Trials
The goal is to have an evidence development framework that can answer a range of questions simultaneously.

Applied Clinical Trials
The eClinical Forum Risk Based Monitoring Taskforce offers some best practices for ensuring clinical data quality.


Applied Clinical Trials
The use of RBM may be the only way forward as long as the development of medicines relies on clinical testing.

Applied Clinical Trials
Risk-based monitoring ushers in the move from the traditional experimental design.



Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
The next 18 to 24 months may bring profound changes for investigative sites.

Applied Clinical Trials
Trial Design: Risk-based Approaches: Best Practices for Ensuring Clinical Data Quality Regulatory: Clinical Research SOPs Also in this issue: Transparency in Europe Fragmented Study Conduct Landscape Comprehensive Evidence Development