
Applied Clinical Trials
Journal editors and legislators expand the scope of clinical trial registration and results disclosure.

Applied Clinical Trials
Journal editors and legislators expand the scope of clinical trial registration and results disclosure.

Applied Clinical Trials
Big pharma collaborates on routine audits and quality management of third-party service providers.
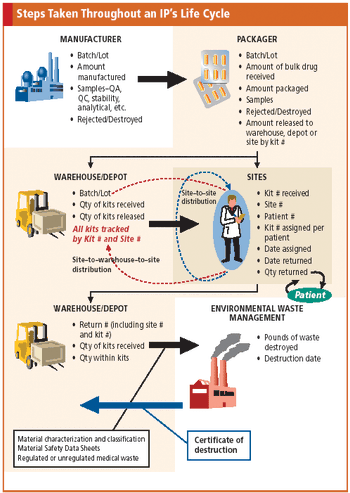
Applied Clinical Trials
Tracking the life cycle of a drug is a complex process that early planning and IVR technology can help ease.
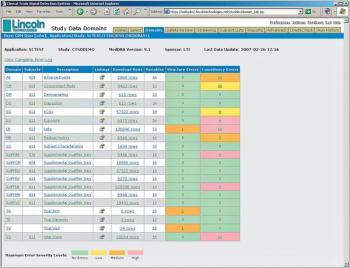
Applied Clinical Trials
Hosted Service Offering Validates Data Prior to FDA Submission to Improve Review Process

Applied Clinical Trials
Fresh and flashy may appeal to the senses, but when it comes to trial software, it's experience that counts.
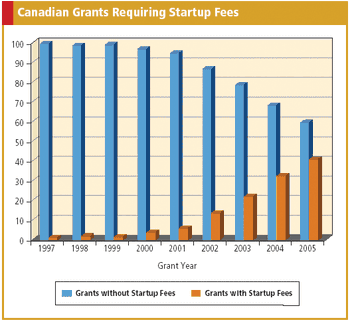
Applied Clinical Trials
Data confirms more sites are asking for separate startup fees and that oncology trials are leading the way.

Applied Clinical Trials
After a long and winding journey, now the real work begins on the EU Regulation for all those involved.

Applied Clinical Trials
Why strategy is the cornerstone of success: Clearly establishing a registry's strategic purpose can save both time and money, eliminating the collection of extraneous data as well as the added burden it spawns.