Applied Clinical Trials
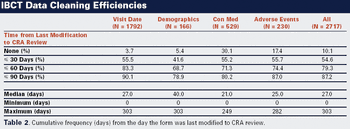
This case study shows how Internet-based clinical trials improve data entry, monitoring, and management.

Applied Clinical Trials
This case study shows how Internet-based clinical trials improve data entry, monitoring, and management.

Applied Clinical Trials
Less stringent requirements in the European Union result in faster medical device approval times.

Applied Clinical Trials
A patient's agreement to take part in a clinical trial is a legal contract, which consumer law requires to be expressed in plain language.

Applied Clinical Trials
From blogs to social networks, the newest Web is redefining the way we use technology.
Applied Clinical Trials
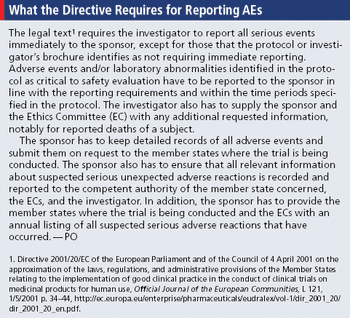
EU helps ease ambiguity of reporting process with recent published guidance.
Applied Clinical Trials
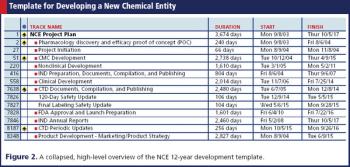
A project plan based on a fully integrated template can position senior management's expectations to minimize surprises.

Applied Clinical Trials
Software vendors can help sponsors ensure clinical trial data are accurate, reliable, and authentic.

Applied Clinical Trials
Why research in Europe has declined since the implementation of the Clinical Trials Directive.
Applied Clinical Trials
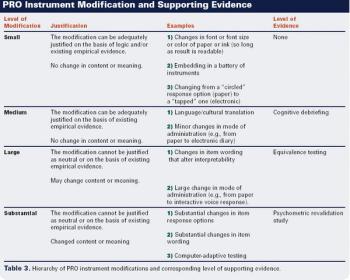
What the agency requires to support the selection of patient reported outcome instruments.

Applied Clinical Trials
Agency seeks to calm critics by improving subject protection, while also streamlining research oversight.
