Applied Clinical Trials
Who better to educate a wary public about clinical research than the industry's own?

Applied Clinical Trials
Who better to educate a wary public about clinical research than the industry's own?
Applied Clinical Trials
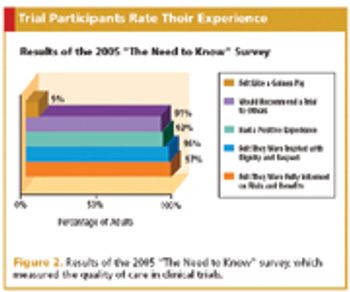
To improve enrollment in oncology trials, one patient advocate group went straight to the source.

Applied Clinical Trials
New to the trials scene, many up-and-coming European firms are seeking the expertise of CROs they can trust.

Applied Clinical Trials
Today's CRAs must redefine their roles in the face of changing industry expectations and new technologies.

Applied Clinical Trials
New regulation provides the EU with a solid framework to stimulate R&D for children's medicines.

Applied Clinical Trials
High-quality programs needed to protect subjects, improve data, harmonize global trials, and manage costs.

Applied Clinical Trials
How a good plan and hot lunch can spell success for sponsors. Attention to detail can help boost dwindling subject enrollment and retention, saving both time and money.
Applied Clinical Trials
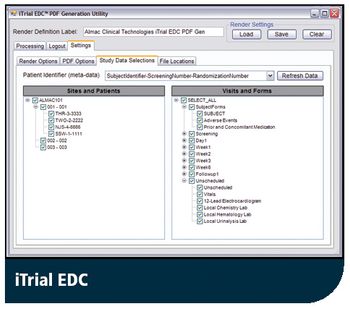
Latest iTrial EDC Includes New PDF Generation Tool to Improve Audit Trail Function

Applied Clinical Trials
Shorter Study Start-Up Times Targeted with Site Assessment Process Tool