Applied Clinical Trials-07-01-2005


Applied Clinical Trials
Recent events highlight the need to clarify rules and policies for pediatric studies.
Applied Clinical Trials
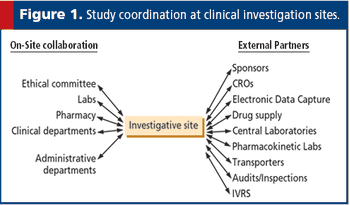
European law now gives prominence to the suitability of investigators and the quality of facilities, two important clinical trial issues.
Applied Clinical Trials
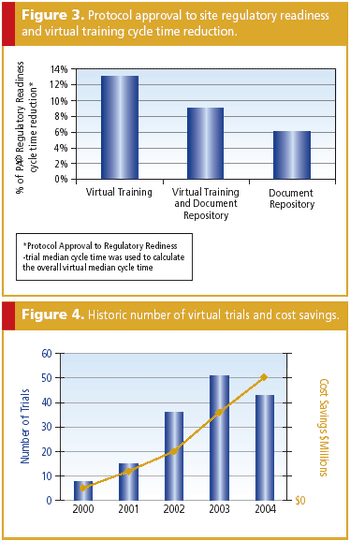
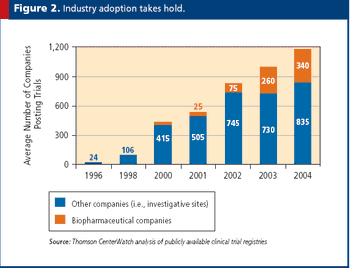
Virtual tools and processes are a key way to decrease costs while increasing quality.

Applied Clinical Trials
The country is in the forefront of European Union nations prepared to explore the brave new world of post-CTD research.
Applied Clinical Trials
Why it is imperative for government and commercial trial publishers to collaborate in addressing this issue faster.

Applied Clinical Trials
Years of debate about pediatric research in the EU may finally end up going somewhere.

Applied Clinical Trials
Following the model of the home health care industry might be the answer to high subject dropout rates in clinical trials.

Applied Clinical Trials
True translation specialists do more than just interpret your words. They interpret your intentions as well.
