Applied Clinical Trials
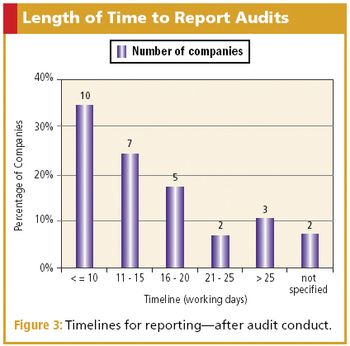
CQA benchmark survey finds companies use a similar method to conduct and report audits.

Applied Clinical Trials
CQA benchmark survey finds companies use a similar method to conduct and report audits.

Applied Clinical Trials
Rethinking the current pharmaceutical model

Applied Clinical Trials
Common barriers to enrollment can be overcome by using targeted recruitment strategies.
Applied Clinical Trials
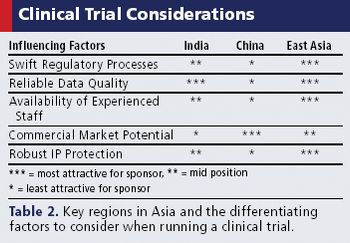
Rapid recruitment, potential cost savings, and investigative sites are just a few of the factors attracting sponsors to the region.

Applied Clinical Trials
A single, industry-wide standard could streamline the clinical trial information management process.

Applied Clinical Trials
Software as a service might be the future of computing, but it won't reside on desktops.
Applied Clinical Trials
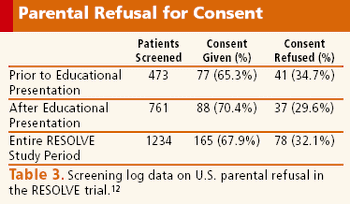
A successful investigative team shares their ideas on improving enrollment in children's research.

Applied Clinical Trials
Electronic health records are a viable alternative to today's subject recruitment methods.
Applied Clinical Trials
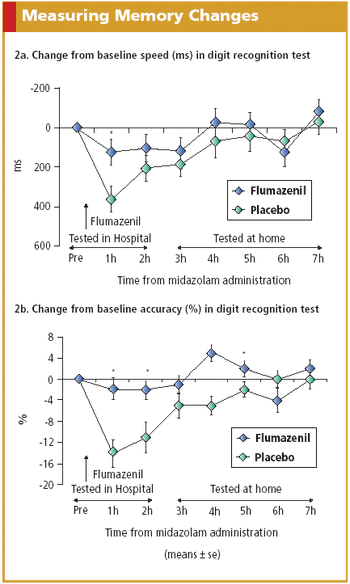
Electronic solutions such as IVR systems, PDAs, and digital pens exhibit advantages over paper and pencil in PRO data collection.

Applied Clinical Trials
Study findings show class attendance improves the outcome of CRA audits.


Applied Clinical Trials
With outsourcing on the rise, it's time companies reevaluated the role of CROs.

Applied Clinical Trials
A comprehensive list of Web sites that contain trial and registry information for both professionals and potential participants

Applied Clinical Trials
New Q&A guidance attempts to clear up any confusion about new Directive rules.