
Applied Clinical Trials
The importance of applying change management techniques throughout the preparation, planning, and execution of RBM and RBQM approaches.

Applied Clinical Trials
The importance of applying change management techniques throughout the preparation, planning, and execution of RBM and RBQM approaches.

Applied Clinical Trials
Where pharmaceutical interventions for Alzheimer's falls short, nursing care facilities are relying heavily on non-traditional medicines and physical and mental exercise.
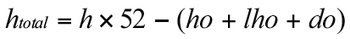
Applied Clinical Trials
Outlining a resource planning and scheduling model, using the example of an analytical department in drug development.

Applied Clinical Trials
President and CEO of Cyclacel, Spiro Rombotis, discusses why targeting cyclin-dependent kinases and the DNA repair pathway could enhance care across various oncology indications.

Applied Clinical Trials
As elections near, politicians in Europe make promises to prioritize the fight against cancer.

Applied Clinical Trials
FDA leaders urge developers, researchers, and research sponsors to help promote policies and programs to streamline clinical research to develop new medical products at reduced costs.

Applied Clinical Trials
What small “nudges” can serve as reminders and relevant motivators to help patients meet their goals, talk to a physician, explore a clinical trial, or stick with a treatment?

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials May 2019 issue in an interactive PDF format.