
Applied Clinical Trials
Negotiating skills can be applied to clinical trial agreements, budgets, and more for effective and fair contracts.

Applied Clinical Trials
Negotiating skills can be applied to clinical trial agreements, budgets, and more for effective and fair contracts.


Applied Clinical Trials
In recent documents and presentations, officials in the OSI in the CDER are highlighting the value of building quality into the design and operation of clinical trials to gain more efficient and effective monitoring and data verification systems.

Applied Clinical Trials
How partnering can speed companion diagnostic development.

Applied Clinical Trials
Sponsor-driven, CRO/recruitment provider collaborations propel cost-efficient study completion.

Applied Clinical Trials
There are scarce or illdefined national and international regulations on quality control and standardization of biorepositories.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
KMR Group


Applied Clinical Trials
The clinical trial debate in the European Parliament is bogged down by rival schools of thought.
Applied Clinical Trials
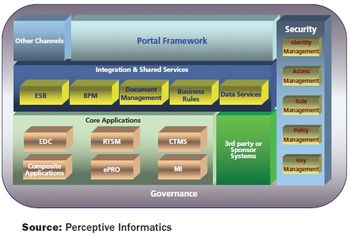
Partnership agreements for CROs and eClinical technology providers can be as beneficial as sponsor/CRO partnerships.

Applied Clinical Trials
Monitoring patient safety is an integral and critical part of the clinical trial process.

Applied Clinical Trials
How Covance has achieved successful outsourcing relationships with pharmaceutical sponsors.

Applied Clinical Trials
A researcher from a top US institution has delivered strong criticism of Roche's promise to release full clinical study reports as part of its data transparency policy, signaling an escalation of the battle over publication of clinical trial results.
Applied Clinical Trials
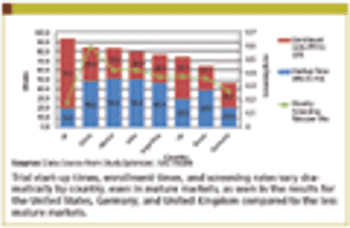
Current commercial forces are working to accelerate the adoption of adaptive monitoring designs.
Applied Clinical Trials
IMS Health

Applied Clinical Trials
A view on redefining key roles across the evolving clinical development landscape.

Applied Clinical Trials

Applied Clinical Trials
CRO/Sponsor: Strategic Partnerships in Trial Outsourcing Trial Design: The Future of Clinical Monitoring Project Management: Negotiating Effective Agreements and Budgets Also in this issue: Trial Debate in European Parliament Redefining Key Roles Biospecimen Storage