
Applied Clinical Trials
The Drug Information Association hosted its 20th Annual European meeting in Barcelona, Spain, where industry experts discussed topics at the forefront of European pharma.

Applied Clinical Trials
The Drug Information Association hosted its 20th Annual European meeting in Barcelona, Spain, where industry experts discussed topics at the forefront of European pharma.
Applied Clinical Trials
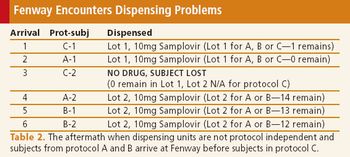
When done right, pooling clinical supplies can increase efficiency and contain costs, but limitations exist.

Applied Clinical Trials
How one patient community on the Web is single-handedly forging new ground in the clinical trials world.
Applied Clinical Trials
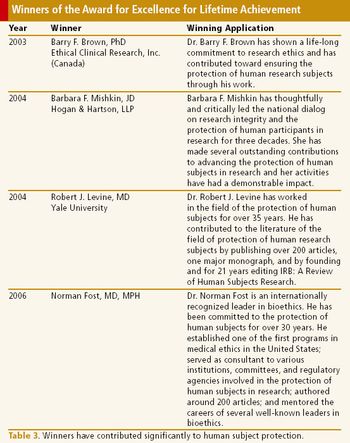
Award for excellence in human research protection is the incentive for better oversight and regulations.

Applied Clinical Trials
Ketek probe raises questions about research oversight by FDA, sponsors, and investigators.

Applied Clinical Trials
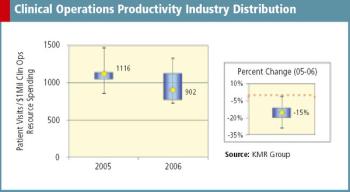
A recent survey from KMR Group shows a 15% decline in clinical operations productivity since 2005.

Applied Clinical Trials
A recent survey uncovers key criteria that influence a sponsor's decision when selecting a CRO.

Applied Clinical Trials
For the first time n a Phase 1 trial, 3D animation is used for informed consent.

Applied Clinical Trials
Europe aims to create new regulations regarding hematological malignancies.
Applied Clinical Trials
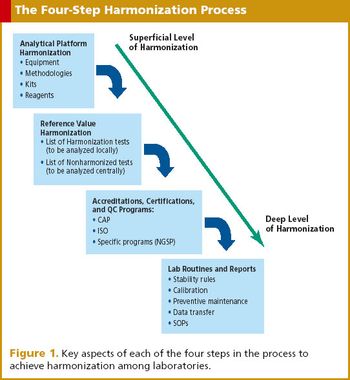
With globalization of trials comes the difficulties of sample logistics. Enter the central laboratory model.

Applied Clinical Trials
Dr. Steve Dodsworth, director of molecular genetic services for Tepnel Research Products & Services, assesses that with molecular insight comes the opportunity to counter disease development and progression.
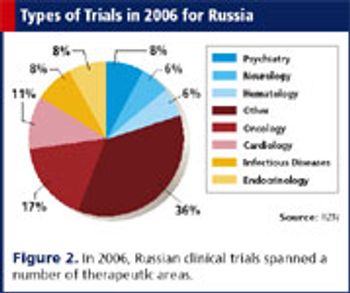
Applied Clinical Trials
Since 1993, investments from big pharma have helped develop a solid infrastructure favorable to CROs.

Applied Clinical Trials
A look at the latest industry news

Applied Clinical Trials
Janet Woodcock is welcomed back as the director of the Center for Drug Evaluation and Research (CDER).

Applied Clinical Trials
New regulations prompt the EU to request more info from sponsors on clinical trial applications.