
Applied Clinical Trials
Personal integrity is what we need to insist on as the finality of good clinical practice.

Applied Clinical Trials
Personal integrity is what we need to insist on as the finality of good clinical practice.
Applied Clinical Trials
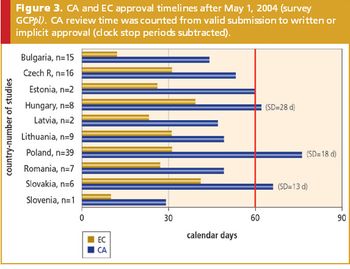
The impact of the EU Clinical Trials Directive on current clinical research practices

Applied Clinical Trials
A system of checks and examinations that helps ensure the quality of clinical trials.

Applied Clinical Trials
A story about 1/2-inch holes and the power of liquid soap

Applied Clinical Trials
FDA seeks to reduce clinical research failures and make drug labeling more useful.

Applied Clinical Trials
A common set of rules for ensuring a two-way flow of information and addressing subjects' needs.

Applied Clinical Trials
The European pharma industry struggles to regain its former prowess in R&D.

Applied Clinical Trials
To clear a pipeline bottleneck, this Sponsor and CRO worked together as a single team.

Applied Clinical Trials
Creating order from information chaos with the help of the Semantic Web.

Applied Clinical Trials
Wyeth's positive experience with partnerships is a good example of R&D progress for the industry.

Applied Clinical Trials
When negative results arise, novel analysis packages help speed the decision to move forward or pull the plug on a new drug.