Applied Clinical Trials
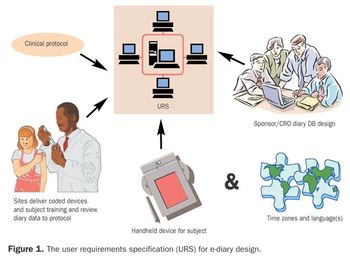
Subject diaries are used in about one-fourth of all clinical trials to collect data on primary and secondary endpoints.1 Part 1 of this four-part series on developing and implementing subject diaries compared and contrasted traditional paper and electronic diaries.2
